It takes a village: channeling fatty acid metabolism and triacylglycerol formation via protein interactomes
- PMID: 30683668
- PMCID: PMC6399496
- DOI: 10.1194/jlr.S091843
It takes a village: channeling fatty acid metabolism and triacylglycerol formation via protein interactomes
Abstract
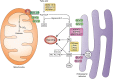
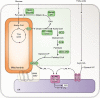
Diet, hormones, gene transcription, and posttranslational modifications control the hepatic metabolism of FAs; metabolic dysregulation causes chronic diseases, including cardiovascular disease, and warrants exploration into the mechanisms directing FA and triacylglycerol (TAG) synthesis and degradation. Long-chain FA metabolism begins by formation of an acyl-CoA by a member of the acyl-CoA synthetase (ACSL) family. Subsequently, TAG synthesis begins with acyl-CoA esterification to glycerol-3-phosphate by a member of the glycerol-3-phosphate acyltransferase (GPAT) family. Our studies of the isoforms ACSL1 and GPAT1 strongly suggest that these proteins are members of larger protein assemblies (interactomes). ACSL1 targeted to the ER interacts with peroxisomal, lipid droplet, and tethering proteins, uncovering a dynamic role for ACSL1 in organelle and lipid droplet interactions. On the outer mitochondrial membrane (OMM), PPARα upregulates ACSL1, which interacts with proteins believed to tether lipid droplets to the OMM. In contrast, GPAT1 is upregulated nutritionally by carbohydrate and insulin in a coordinated sequence of enzyme reactions, from saturated FA formation via de novo lipogenesis to FA esterification by GPAT1 and entry into the TAG biosynthesis pathway. We propose that involved enzymes form a dynamic protein interactome that facilitates esterification and that other lipid-metabolizing pathways will exist in similar physiologically regulated interactomes.
Keywords: acyl-coenzyme A; beta oxidation; de novo lipogenesis; lipid droplets.
Copyright © 2019 Coleman.
Conflict of interest statement
The author declares no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

