Polyadenylation and nuclear export of mRNAs
- PMID: 30683695
- PMCID: PMC6398137
- DOI: 10.1074/jbc.REV118.005594
Polyadenylation and nuclear export of mRNAs
Abstract
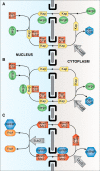
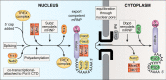
In eukaryotes, the separation of translation from transcription by the nuclear envelope enables mRNA modifications such as capping, splicing, and polyadenylation. These modifications are mediated by a spectrum of ribonuclear proteins that associate with preRNA transcripts, coordinating the different steps and coupling them to nuclear export, ensuring that only mature transcripts reach the cytoplasmic translation machinery. Although the components of this machinery have been identified and considerable functional insight has been achieved, a number of questions remain outstanding about mRNA nuclear export and how it is integrated into the nuclear phase of the gene expression pathway. Nuclear export factors mediate mRNA transit through nuclear pores to the cytoplasm, after which these factors are removed from the mRNA, preventing transcripts from returning to the nucleus. However, as outlined in this review, several aspects of the mechanism by which transport factor binding and release are mediated remain unclear, as are the roles of accessory nuclear components in these processes. Moreover, the mechanisms by which completion of mRNA splicing and polyadenylation are recognized, together with how they are coordinated with nuclear export, also remain only partially characterized. One attractive hypothesis is that dissociating poly(A) polymerase from the cleavage and polyadenylation machinery could signal completion of mRNA maturation and thereby provide a mechanism for initiating nuclear export. The impressive array of genetic, molecular, cellular, and structural data that has been generated about these systems now provides many of the tools needed to define the precise mechanisms involved in these processes and how they are integrated.
Keywords: RNA binding protein; RNA helicase; RNA splicing; gene expression pathway; nuclear pore; nuclear posttranscriptional modification; nuclear transport; polyadenylation; ribonuclear protein (RNP); spliceosome.
© 2019 Stewart.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
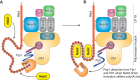
Figures
Similar articles
-
Integration of mRNP formation and export.Cell Mol Life Sci. 2017 Aug;74(16):2875-2897. doi: 10.1007/s00018-017-2503-3. Epub 2017 Mar 17. Cell Mol Life Sci. 2017. PMID: 28314893 Free PMC article. Review.
-
Structural basis of mRNA maturation: Time to put it together.Curr Opin Struct Biol. 2022 Aug;75:102431. doi: 10.1016/j.sbi.2022.102431. Epub 2022 Aug 2. Curr Opin Struct Biol. 2022. PMID: 35930970 Review.
-
Nucleocytosolic mRNA transport in plants: export factors and their influence on growth and development.J Exp Bot. 2019 Aug 7;70(15):3757-3763. doi: 10.1093/jxb/erz173. J Exp Bot. 2019. PMID: 30972423
-
Distinct Functions of the Cap-Binding Complex in Stimulation of Nuclear mRNA Export.Mol Cell Biol. 2019 Apr 2;39(8):e00540-18. doi: 10.1128/MCB.00540-18. Print 2019 Apr 15. Mol Cell Biol. 2019. PMID: 30745412 Free PMC article.
-
Structure-function relationships in the Nab2 polyadenosine-RNA binding Zn finger protein family.Protein Sci. 2019 Mar;28(3):513-523. doi: 10.1002/pro.3565. Epub 2019 Jan 16. Protein Sci. 2019. PMID: 30578643 Free PMC article. Review.
Cited by
-
RNA-binding proteins in bone pathophysiology.Front Cell Dev Biol. 2024 Jun 20;12:1412268. doi: 10.3389/fcell.2024.1412268. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38966428 Free PMC article. Review.
-
The Role of Tau Proteoforms in Health and Disease.Mol Neurobiol. 2023 Sep;60(9):5155-5166. doi: 10.1007/s12035-023-03387-8. Epub 2023 Jun 2. Mol Neurobiol. 2023. PMID: 37266762 Review.
-
mRNA alternative polyadenylation (APA) in regulation of gene expression and diseases.Genes Dis. 2021 Oct 18;10(1):165-174. doi: 10.1016/j.gendis.2021.09.005. eCollection 2023 Jan. Genes Dis. 2021. PMID: 37013028 Free PMC article. Review.
-
mRNA-Based Vaccines and Mode of Action.Curr Top Microbiol Immunol. 2022;440:1-30. doi: 10.1007/82_2020_230. Curr Top Microbiol Immunol. 2022. PMID: 33591423 Review.
-
Introns and Their Therapeutic Applications in Biomedical Researches.Iran J Biotechnol. 2023 Oct 1;21(4):e3316. doi: 10.30498/ijb.2023.334488.3316. eCollection 2023 Oct. Iran J Biotechnol. 2023. PMID: 38269198 Free PMC article. Review.
References
-
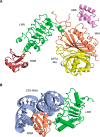
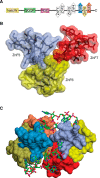
- Kim S. J., Fernandez-Martinez J., Nudelman I., Shi Y., Zhang W., Raveh B., Herricks T., Slaughter B. D., Hogan J. A., Upla P., Chemmama I. E., Pellarin R., Echeverria I., Shivaraju M., Chaudhury A. S., et al. (2018) Integrative structure and functional anatomy of a nuclear pore complex. Nature 555, 475–482 10.1038/nature26003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

