Exploiting Prophage-Mediated Lysis for Biotherapeutic Release by Lactobacillus reuteri
- PMID: 30683744
- PMCID: PMC6498169
- DOI: 10.1128/AEM.02335-18
Exploiting Prophage-Mediated Lysis for Biotherapeutic Release by Lactobacillus reuteri
Abstract
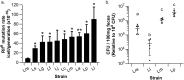
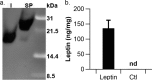
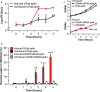
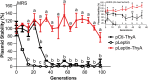
Lactobacillus reuteri has the potential to be developed as a microbial therapeutic delivery platform because of an established safety profile, health-promoting properties, and available genome editing tools. Here, we show that L. reuteri VPL1014 exhibits a low mutation rate compared to other Gram-positive bacteria, which we expect will contribute to the stability of genetically modified strains. VPL1014 encodes two biologically active prophages, which are induced during gastrointestinal transit. We hypothesized that intracellularly accumulated recombinant protein can be released following bacteriophage-mediated lysis. To test this, we engineered VPL1014 to accumulate leptin, our model protein, inside the cell. In vitro prophage induction of recombinant VPL1014 released leptin into the extracellular milieu, which corresponded to bacteriophage production. We also employed a plasmid system that does not require antibiotic in the growth medium for plasmid maintenance. Collectively, these data provide new avenues to exploit native prophages to deliver therapeutic molecules.IMPORTANCE Lactic acid bacteria (LAB) have been explored as potential biotherapeutic vehicles for the past 20 years. To secrete a therapeutic in the extracellular milieu, one typically relies on the bacterial secretion pathway, i.e., the Sec pathway. Overexpression of a secreted protein can overload the secretory pathway and impact the organism's fitness, and optimization of the signal peptide is also required to maximize the efficiency of the release of mature protein. Here, we describe a previously unexplored approach to release therapeutics from the probiotic Lactobacillus reuteri We demonstrate that an intracellularly accumulated recombinant protein is released following prophage activation. Since we recently demonstrated that prophages are activated during gastrointestinal transit, we propose that this method will provide a straightforward and efficient approach to deliver therapeutics in vivo.
Keywords: Lactobacillus reuteri; bacteriophage; leptin; microbial delivery; probiotic; prophage; therapeutic.
Copyright © 2019 Alexander et al.
Figures
References
-
- Keles G, Demirci U. 2011. The effect of homofermentative and heterofermentative lactic acid bacteria on conservation characteristics of baled triticale-Hungarian vetch silage and lamb performance. Anim Feed Sci Technol 164:21–28. doi: 10.1016/j.anifeedsci.2010.11.017. - DOI
-
- Ayad EHE, Verheul A, Wouters JTM, Smit G. 2002. Antimicrobial-producing wild lactococci isolated from artisanal and non-dairy origins. Int Dairy J 12:145–150. doi: 10.1016/S0958-6946(01)00133-9. - DOI
-
- Mattila-Sandholm T, Mättö J, Saarela M. 1999. Lactic acid bacteria with health claims—interactions and interference with gastrointestinal flora. Int Dairy J 9:25–35. doi: 10.1016/S0958-6946(99)00041-2. - DOI
-
- Mercenier A, Müller-Alouf H, Grangette C. 2000. Lactic acid bacteria as live vaccines. Curr Issues Mol Biol 2:17–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

