Newborn Screening for Severe Combined Immunodeficiency and T-cell Lymphopenia in California, 2010-2017
- PMID: 30683812
- PMCID: PMC6361357
- DOI: 10.1542/peds.2018-2300
Newborn Screening for Severe Combined Immunodeficiency and T-cell Lymphopenia in California, 2010-2017
Abstract
Objectives: Newborn screening for severe combined immunodeficiency (SCID) was instituted in California in 2010. In the ensuing 6.5 years, 3 252 156 infants in the state had DNA from dried blood spots assayed for T-cell receptor excision circles (TRECs). Abnormal TREC results were followed-up with liquid blood testing for T-cell abnormalities. We report the performance of the SCID screening program and the outcomes of infants who were identified.
Methods: Data that were reviewed and analyzed included demographics, nursery summaries, TREC and lymphocyte flow-cytometry values, and available follow-up, including clinical and genetic diagnoses, treatments, and outcomes.
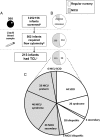
Results: Infants with clinically significant T-cell lymphopenia (TCL) were successfully identified at a rate of 1 in 15 300 births. Of these, 50 cases of SCID, or 1 in 65 000 births (95% confidence interval 1 in 51 000-1 in 90 000) were found. Prompt treatment led to 94% survival. Infants with non-SCID TCL were also identified, diagnosed and managed, including 4 with complete DiGeorge syndrome who received thymus transplants. Although no cases of typical SCID are known to have been missed, 2 infants with delayed-onset leaky SCID had normal neonatal TREC screens but came to clinical attention at 7 and 23 months of age.
Conclusions: Population-based TREC testing, although unable to detect immune defects in which T cells are present at birth, is effective for identifying SCID and clinically important TCL with high sensitivity and specificity. The experience in California supports the rapid, widespread adoption of SCID newborn screening.
Copyright © 2019 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Markert developed technology for RVT-802, which has been licensed to Enzyvant Therapeutics GmbH. Dr Markert has received royalties from Enzyvant. Portions of Dr Markert and her research team’s salaries are being paid by the funding from Enzyvant. If the technology is commercially successful in the future, Dr Markert and Duke University may benefit financially. Dr Naides is employed by Quest Diagnostics. Donald Kohn is an inventor of a lentiviral vector for gene therapy of adenosine deaminase severe combined immunodeficiency, which Orchard Therapeutics Ltd has licensed from the University of California Regents; Dr Kohn is a consultant to Orchard Therapeutics Ltd and a member of its Scientific Advisory Board. Dr Cowan serves on the Scientific Advisory Boards for Homology Medicine, Inc, and Exogen, Inc, and on the Data and Safety Monitoring Board for bluebird bio, Inc. Dr Puck discloses spousal employment at a clinical DNA sequencing company, Invitae; the other authors have indicated they have no potential conflicts of interest to disclose.
Figures

References
-
- Buckley RH, Schiff RI, Schiff SE, et al. . Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130(3):378–387 - PubMed
-
- Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115(2):391–398 - PubMed
-
- Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–878 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

