Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for the treatment of in-stent restenosis of the femoropopliteal arteries
- PMID: 30684445
- PMCID: PMC6353053
- DOI: 10.1002/14651858.CD012510.pub2
Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for the treatment of in-stent restenosis of the femoropopliteal arteries
Abstract
Background: Stents are placed in the femoropopliteal arteries for numerous reasons, such as atherosclerotic disease, the need for dissection, and perforation of the arteries, and can become stenosed with the passage of time. When a stent develops a flow-limiting stenosis, this process is known as "in-stent stenosis." It is thought that in-stent restenosis is caused by a process known as "intimal hyperplasia" rather than by the progression of atherosclerotic disease. Management of in-stent restenosis may include performing balloon angioplasty, deploying another stent within the stenosed stent to force it open, and creating a bypass to deliver blood around the stent. The role of drug-eluting technologies, such as drug-eluting balloons (DEBs), in the management of in-stent restenosis is unclear. Drug-eluting balloons might function by coating the inside of stenosed stents with cytotoxic chemicals such as paclitaxel and by inhibiting the hyperplastic processes responsible for in-stent restenosis. It is important to perform this systematic review to evaluate the efficacy of DEB because of the potential for increased expenses associated with DEBs over uncoated balloon angioplasty, also known as plain old balloon angioplasty (POBA).
Objectives: To assess the safety and efficacy of DEBs compared with uncoated balloon angioplasty in people with in-stent restenosis of the femoropopliteal arteries as assessed by criteria such as amputation-free survival, vessel patency, target lesion revascularization, binary restenosis rate, and death. We define "in-stent restenosis" as 50% or greater narrowing of a previously stented vessel by duplex ultrasound or angiography.
Search methods: The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, and CINAHL databases and the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to November 28, 2017. Review authors also undertook reference checking to identify additional studies.
Selection criteria: We included all randomized controlled trials that compared DEBs versus uncoated balloon angioplasty for treatment of in-stent restenosis in the femoropopliteal arteries.
Data collection and analysis: Two review authors (AK, WA) independently selected appropriate trials and performed data extraction, assessment of trial quality, and data analysis. The senior review author (AD) adjudicated any disagreements.
Main results: Three trials that randomized a combined total of 263 participants met the review inclusion criteria. All three trials examined the treatment of symptomatic in-stent restenosis within the femoropopliteal arteries. These trials were carried out in Germany and Austria and used paclitaxel as the agent in the drug-eluting balloons. Two of the three trials were industry sponsored. Two companies manufactured the drug-eluting balloons (Eurocor, Bonn, Germany; Medtronic, Fridley, Minnesota, USA). The trials examined both anatomical and clinical endpoints. We noted heterogeneity in the frequency of bailout stenting deployment between studies as well as in the dosage of paclitaxel applied by the DEBs. Using GRADE assessment criteria, we determined that the certainty of evidence presented was very low for the outcomes of amputation, target lesion revascularization, binary restenosis, death, and improvement of one or more Rutherford categories. Most participants were followed up to 12 months, but one trial followed participants for up to 24 months.Trial results show no difference in the incidence of amputation between DEBs and uncoated balloon angioplasty. DEBs showed better outcomes for up to 24 months for target lesion revascularization (odds ratio (OR) 0.05, 95% confidence Interval (CI) 0.00 to 0.92 at six months; OR 0.24, 95% CI 0.08 to 0.70 at 24 months) and at six and 12 months for binary restenosis (OR 0.28, 95% CI 0.14 to 0.56 at six months; OR 0.34, 95% CI 0.15 to 0.76 at 12 months). Participants treated with DEBs also showed improvement of one or more Rutherford categories at six and 12 months (OR 1.81, 95% CI 1.02 to 3.21 at six months; OR 2.08, 95% CI 1.13 to 3.83 at 12 months). Data show no clear differences in death between DEBs and uncoated balloon angioplasty. Data were insufficient for subgroup or sensitivity analyses to be conducted.
Authors' conclusions: Based on a meta-analysis of three trials with 263 participants, evidence suggests an advantage for DEBs compared with uncoated balloon angioplasty for anatomical endpoints such as target lesion revascularization (TLR) and binary restenosis, and for one clinical endpoint - improvement in Rutherford category post intervention for up to 24 months. However, the certainty of evidence for all these outcomes is very low due to the small number of included studies and participants and the high risk of bias in study design. Adequately powered and carefully constructed randomized controlled trials are needed to adequately investigate the role of drug-eluting technologies in the management of in-stent restenosis.
Conflict of interest statement
AK: none known. WAJ: none known. GP: received consultancy fees from WL Gore for acting as a reviewer of complications for a study involving an endovascular device and for support for speaking/lectures. He has received a grant from Gore for creation of a database for endovascular aneurysm repair research and from Abbott for development of a database for peripheral endovascular procedures. He has received honoraria from WL Gore and Medtronic for lectures and for speaking. DK: none known. TF: none known. Reports that his host institution, the University of Toronto Division of Vascular Surgery, receives academic support from several industry partners (Cook Medical, Medtronic, Liva Nova, CHS, Lemaitre, Gore) in the form of unrestricted educational grants. DR: received consultancy fees for lectures around dialysis interventions and support for a live case course from CR Bard. RN: has declared that he received payment for roles within the Scientific Advisory Boards of Graftworx and WL Gore; and from Fresenius for presenting an educational program for podiatric audiences and for legal defence malpractice work. He has no financial relationships related to the topic of this review. AD: none known.
Figures

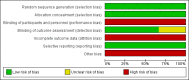











Update of
References
References to studies included in this review
FAIR {published data only (unpublished sought but not used)}
-
- Krankenberg H. FAIR drug eluting balloon vs. PTA for superficial femoral artery in‐stent restenosis 12‐month results. The Leipzig Interventional Course. Leipzig 28‐31 January 2014.
-
- Krankenberg H, Tübler T, Ingwersen M, Schlüter M, Scheinert D, Blessing E, et al. Drug‐coated balloon versus standard balloon for superficial femoral artery in‐stent restenosis: the randomized femoral artery in‐stent restenosis (FAIR) trial. Circulation 2015;132(23):2230‐6. - PubMed
-
- NCT01305070. Standard balloon angioplasty versus angioplasty with a paclitaxel‐eluting balloon for Femoral Artery In‐stent Restenosis (FAIR). clinicaltrials.gov/ct2/show/NCT01305070 (date first received February 28, 2011).
ISAR‐PEBIS {published data only (unpublished sought but not used)}
-
- NCT01083394. Paclitaxel eluting balloon and conventional balloon for in‐stent restenosis of the superficial femoral artery (ISAR‐PEBIS). clinicaltrials.gov/ct2/show/NCT01083394 (date first received March 9, 2010).
-
- Ott I, Cassese S, Groha P, Steppich B, Voll F, Hadamitzky M, et al. ISAR‐PEBIS (paclitaxel‐eluting balloon versus conventional balloon angioplasty for in‐stent restenosis of superficial femoral artery): a randomized trial. Journal of the American Heart Association 2017;6(7):pii: e006321. - PMC - PubMed
PACUBA {published data only (unpublished sought but not used)}
-
- Kinstner CM, Lammer J, Willfort‐Ehringer A, Matzek W, Gschwandtner M, Javor D, et al. Paclitaxel‐eluting balloon versus standard balloon angioplasty in in‐stent restenosis of the superficial femoral and proximal popliteal artery: 1‐year results of the PACUBA trial. JACC Cardiovascular Interventions 2016;9(13):1386‐92. - PubMed
-
- Kinstner CM, Schoder M, Funovics MA, Willfort‐Ehringer A, Gschwandtner M, Ristl R, et al. A monocenter randomized clinical trial of PAClitaxel drUgeluting Balloon versus standard percutaneous transluminal Angioplasty to reduce restenosis in patients with in‐stent stenoses in the superficial femoral and proximal popliteal artery (PACUBA I Trial). Cardiovascular and Interventional Radiology 2015;38(3 Suppl 1):S288.
-
- NCT01247402. Paclitaxel balloon versus standard balloon in in‐stent restenoses of the superficial femoral artery (PACUBA I Trial) (PACUBA 1). clinicaltrials.gov/ct2/show/NCT01247402 (date first received November 24, 2010).
References to studies excluded from this review
Bosiers 2015 {published data only}
-
- Bosiers M, Deloose K, Callaert J, Verbist J, Hendriks J, Lauwers P, et al. Superiority of stent‐grafts for in‐stent restenosis in the superficial femoral artery: twelve‐month results from a multicenter randomized trial. Journal of Endovascular Therapy 2015;22(1):1‐10. - PubMed
ConSeQuent {published data only}
-
- Albrecht T, Meyer D, Müller‐Hülsbeck S, Ott I, Redlich U, Ricke J, et al. Preliminary angiographic and clinical 6‐month results of the CONSEQUENT trial. The Leipig Interventional Course. Leipzig 26‐29 January 2016.
-
- NCT01970579. Clinical trial on peripheral arteries treated with SeQuent® Please P Paclitaxel coated balloon catheter (ConSeQuent). clinicaltrials.gov/ct2/show/NCT01970579 (date first received October 28, 2013).
DEBATE‐ISR {published data only}
-
- Liistro F, Angioli P, Porto I, Ricci L, Ducci K, Grotti S, et al. Paclitaxel‐eluting balloon vs. standard angioplasty to reduce recurrent restenosis in diabetic patients with in‐stent restenosis of the superficial femoral and proximal popliteal arteries: the DEBATE‐ISR study. Journal of Endovascular Therapy 2014;21(1):1‐8. - PubMed
EXCITE ISR {published data only}
-
- Dippel EJ, Makam P, Kovach R, George JC, Patlola R, Metzger DC, et al. Randomized controlled study of excimer laser atherectomy for treatment of femoropopliteal in‐stent restenosis: initial results from the EXCITE ISR trial (EXCImer Laser Randomized Controlled Study for Treatment of FemoropopliTEal In‐Stent Restenosis). JACC.Cardiovascular interventions 2015;8:92‐101. - PubMed
-
- Dippel EJ, Makam P, Kovach RC, George JC, et al. EXCITE ISR: a prospective, randomized controlled trial of Excimer laser atherectomy vs balloon angioplasty for the treatment of femoropopliteal in‐stent restenosis. Journal of the American College of Cardiology 2014; Vol. 64, issue Suppl 11.
-
- NCT01330628. Randomized study of laser and balloon angioplasty versus balloon angioplasty to treat peripheral in‐stent restenosis (EXCITE ISR). clinicaltrials.gov/ct2/show/NCT01330628 (date first received April 7, 2011).
NCT00481780 {published data only}
-
- NCT00481780. Conventional balloon angioplasty vs. cutting balloon angioplasty for treatment of femoropopliteal artery in‐stent restenosis ‐ a randomized controlled pilot trial. clinicaltrials.gov/ct2/show/NCT00481780 (date first received June 4, 2007).
NCT02832024 {published data only}
-
- NCT02832024. Clinical study of stent versus direct atherectomy versus angioplasty to treat lower limb in‐stent restenosis. clinicaltrials.gov/ct2/show/NCT02832024 (date first received July 13, 2016).
RELINE {published data only}
-
- Deloose K. RELINE randomized clinical trial: Viabahn covered stents vs PTA. The Leipzig Interventional Course. Leipzig 28‐31 January 2014.
-
- NCT01108861. GORE VIABAHN® versus plain old balloon angioplasty (POBA) for superficial femoral artery (SFA) in‐stent restenosis (RELINE). clinicaltrials.gov/ct2/show/NCT01108861 (date first received April 22, 2010).
References to ongoing studies
Copa Cabana {published data only (unpublished sought but not used)}
-
- NCT01594684. Cotavance™ paclitaxel‐coated balloon versus uncoated balloon angioplasty for treatment of in‐stent restenosis in SFA and popliteal arteries. clinicaltrials.gov/ct2/show/results/NCT01594684 (date first received May 9, 2012).
TRC‐14004848 {published data only (unpublished sought but not used)}
-
- ChiCTR‐TRC‐14004848. Drug‐eluting balloon versus standard balloon angioplasty for the treatment of in‐stent restenosis in the femoral‐popliteal artery: a randomized, controlled prospective study. chictr.org.cn/hvshowproject.aspx?id=11037 (date first received May 1, 2015).
Additional references
Alfonso 2014
-
- Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in‐stent restenosis. Journal of the American College of Cardiology 2014;63(24):2659‐73. - PubMed
Ali 2012
-
- Ali FN, Carman TL. Medical management for chronic atherosclerotic peripheral arterial disease. Drugs 2012;72(16):2073‐85. - PubMed
Atkins 2004
Canaud 2014
-
- Canaud L, Ozdemir BA, Belli AM, Loftus IM, Thompson MM, Hinchliffe RJ. Infrainguinal angioplasty with drug‐eluting stents and balloons. Journal of Vascular Surgery 2014;59(6):1721‐36. - PubMed
Cassese 2017
-
- Cassese S, Ndrepepa G, Kufner S, Byrne RA, Giacoppo D, Ott I, et al. Drug‐coated balloon angioplasty for in‐stent restenosis of femoropopliteal arteries: a meta‐analysis. EuroIntervention 2017;13(4):483‐9. - PubMed
Conte 2015
-
- Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. Journal of Vascular Surgery 2015;61(3 Suppl):2S‐41S. - PubMed
Criqui 2015
-
- Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circulation Research 2015;116(9):1509‐26. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2003
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Indermuehle 2013
-
- Indermuehle A, Bahl R, Lansky AJ, Froehlich GM, Knapp G, Timmis A, et al. Drug‐eluting balloon angioplasty for in‐stent restenosis: a systematic review and meta‐analysis of randomised controlled trials. Heart 2013;99(5):327‐33. - PubMed
Marupudi 2007
-
- Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opinion on Drug Safety 2007;6(5):609‐21. - PubMed
McDermott 2001
-
- McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. Journal of the American Medical Association 2001;286(13):1599‐606. - PubMed
Mills 2014
-
- Mills JL, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery lower extremity threatened limb classification system: risk stratification based on Wound, Ischemia, and foot Infection (WIfI). Journal of Vascular Surgery 2014;59(1):220‐34. - PubMed
OHTAC 2010
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Singh 2014
-
- Singh GD, Armstrong EJ, Laird JR. Femoropopliteal in‐stent restenosis: current treatment strategies. Journal of Cardiovascular Surgery 2014;55(3):325‐33. - PubMed
Stary 1994
-
- Stary H, Chandler AB, Glagov S, Guyton JR, Insull W, Rosenfeld ME, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994;89(5):2462‐78. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Waksman 2009
-
- Waksman R, Pakala R. Drug‐eluting balloon: the comeback kid?. Circulation Cardiovascular Interventions 2009;2(4):352‐8. - PubMed
Wu 2017
-
- Wu R, Li Z, Wang M, Chang G, Yao C, Wang S. Paclitaxel‐coated versus uncoated balloon angioplasty for femoropopliteal artery in‐stent restenosis. International Journal of Surgery 2017;42:72‐82. - PubMed
References to other published versions of this review
Kayssi 2017
-
- Kayssi A, Al‐Jundi W, Papia G, Kucey DS, Forbes T, Rajan DK, et al. Drug‐eluting balloon angioplasty versus uncoated balloon angioplasty for the treatment of in‐stent restenosis of the femoropopliteal arteries. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012510] - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

