Potential for Heightened Sulfur-Metabolic Capacity in Coastal Subtropical Microalgae
- PMID: 30684492
- PMCID: PMC6348204
- DOI: 10.1016/j.isci.2018.12.035
Potential for Heightened Sulfur-Metabolic Capacity in Coastal Subtropical Microalgae
Abstract
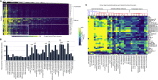
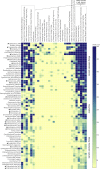
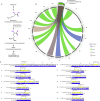
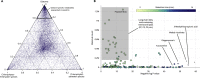
The activities of microalgae support nutrient cycling that helps to sustain aquatic and terrestrial ecosystems. Most microalgal species, especially those from the subtropics, are genomically uncharacterized. Here we report the isolation and genomic characterization of 22 microalgal species from subtropical coastal regions belonging to multiple clades and three from temperate areas. Halotolerant strains including Halamphora, Dunaliella, Nannochloris, and Chloroidium comprised the majority of these isolates. The subtropical-based microalgae contained arrays of methyltransferase, pyridine nucleotide-disulfide oxidoreductase, abhydrolase, cystathionine synthase, and small-molecule transporter domains present at high relative abundance. We found that genes for sulfate transport, sulfotransferase, and glutathione S-transferase activities were especially abundant in subtropical, coastal microalgal species and halophytic species in general. Our metabolomics analyses indicate lineage- and habitat-specific sets of biomolecules implicated in niche-specific biological processes. This work effectively expands the collection of available microalgal genomes by ∼50%, and the generated resources provide perspectives for studying halophyte adaptive traits.
Keywords: Algology; Genomics; Global Nutrient Cycle; Metabolomics.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Alcolombri U., Ben-Dor S., Feldmesser E., Levin Y., Tawfik D.S., Vardi A. MARINE SULFUR CYCLE. Identification of the algal dimethyl sulfide-releasing enzyme: a missing link in the marine sulfur cycle. Science. 2015;348:1466–1469. - PubMed
-
- Arnold H.E., Kerrison P., Steinke M. Interacting effects of ocean acidification and warming on growth and DMS-production in the haptophyte coccolithophore Emiliania huxleyi. Glob. Chang. Biol. 2013;19:1007–1016. - PubMed
LinkOut - more resources
Full Text Sources

