Complex, coordinated and highly regulated changes in placental signaling and nutrient transport capacity in IUGR
- PMID: 30684642
- PMCID: PMC6650384
- DOI: 10.1016/j.bbadis.2018.12.024
Complex, coordinated and highly regulated changes in placental signaling and nutrient transport capacity in IUGR
Abstract
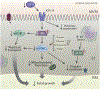
The most common cause of intrauterine growth restriction (IUGR) in the developed world is placental insufficiency, a concept often used synonymously with reduced utero-placental and umbilical blood flows. However, placental insufficiency and IUGR are associated with complex, coordinated and highly regulated changes in placental signaling and nutrient transport including inhibition of insulin and mTOR signaling and down-regulation of specific amino acid transporters, Na+/K+-ATPase, the Na+/H+-exchanger, folate and lactate transporters. In contrast, placental glucose transport capacity is unaltered and Ca2+-ATPase activity and the expression of proteins involved in placental lipid transport are increased in IUGR. These findings are not entirely consistent with the traditional view that the placenta is dysfunctional in IUGR, but rather suggest that the placenta adapts to reduce fetal growth in response to an inability of the mother to allocate resources to the fetus. This new model has implications for the understanding of the mechanisms underpinning IUGR and for the development of intervention strategies.
Keywords: Fetal development; Fetal growth restriction; Human; Maternal-fetal exchange; Placental transport; Syncytiotrophoblast.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures

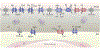

Similar articles
-
Adaptive responses in uteroplacental metabolism and fetoplacental nutrient shuttling and sensing during placental insufficiency.Am J Physiol Endocrinol Metab. 2023 Jun 1;324(6):E556-E568. doi: 10.1152/ajpendo.00046.2023. Epub 2023 Apr 26. Am J Physiol Endocrinol Metab. 2023. PMID: 37126847 Free PMC article.
-
Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet.J Physiol. 2006 Nov 1;576(Pt 3):935-46. doi: 10.1113/jphysiol.2006.116509. J Physiol. 2006. PMID: 16916910 Free PMC article.
-
Adaptive responses to maternal nutrient restriction alter placental transport in ewes.Placenta. 2020 Jul;96:1-9. doi: 10.1016/j.placenta.2020.05.002. Epub 2020 May 7. Placenta. 2020. PMID: 32421527
-
The role of trophoblast nutrient and ion transporters in the development of pregnancy complications and adult disease.Curr Vasc Pharmacol. 2009 Oct;7(4):521-33. doi: 10.2174/157016109789043982. Curr Vasc Pharmacol. 2009. PMID: 19485888 Review.
-
Fetoplacental transport and utilization of amino acids in IUGR--a review.Placenta. 2005 Apr;26 Suppl A:S52-62. doi: 10.1016/j.placenta.2005.01.003. Placenta. 2005. PMID: 15837069 Review.
Cited by
-
Vasoactive Intestinal Peptide induces glucose and neutral amino acid uptake through mTOR signalling in human cytotrophoblast cells.Sci Rep. 2019 Nov 20;9(1):17152. doi: 10.1038/s41598-019-53676-3. Sci Rep. 2019. PMID: 31748639 Free PMC article.
-
Down-regulation of placental Cdc42 and Rac1 links mTORC2 inhibition to decreased trophoblast amino acid transport in human intrauterine growth restriction.Clin Sci (Lond). 2020 Jan 17;134(1):53-70. doi: 10.1042/CS20190794. Clin Sci (Lond). 2020. PMID: 31825077 Free PMC article.
-
Maternal-fetal cross-talk via the placenta: influence on offspring development and metabolism.Development. 2023 Oct 15;150(20):dev202088. doi: 10.1242/dev.202088. Epub 2023 Oct 13. Development. 2023. PMID: 37831056 Free PMC article. Review.
-
Placental Function and the Development of Fetal Overgrowth and Fetal Growth Restriction.Obstet Gynecol Clin North Am. 2021 Jun;48(2):247-266. doi: 10.1016/j.ogc.2021.02.001. Obstet Gynecol Clin North Am. 2021. PMID: 33972064 Free PMC article. Review.
-
Curcumin: Could This Compound Be Useful in Pregnancy and Pregnancy-Related Complications?Nutrients. 2020 Oct 17;12(10):3179. doi: 10.3390/nu12103179. Nutrients. 2020. PMID: 33080891 Free PMC article. Review.
References
-
- Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev 2009; 6 Suppl 3:332–336. - PubMed
-
- Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol 2006; 49:257–269. - PubMed
-
- Froen JF, Gardosi JO, Thurmann A, Francis A, Stray-Pedersen B. Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet Gynecol Scand 2004; 83:801–807. - PubMed
-
- Silver RM. Examining the link between placental pathology, growth restriction, and stillbirth. Best Pract Res Clin Obstet Gynaecol 2018; 49:89–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

