Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf2 mediated antioxidant capacity
- PMID: 30684890
- PMCID: PMC6351233
- DOI: 10.1016/j.redox.2018.101068
Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf2 mediated antioxidant capacity
Abstract
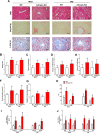
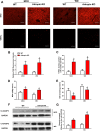
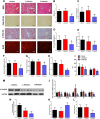
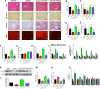
Adropin, a secretory signal peptide, has shown beneficial effects on improving glucose homeostasis and dyslipidemia. However, whether this peptide affects nonalcoholic steatohepatitis (NASH) has remained unclear. In this study, the serum adropin levels, liver injury and oxidative stress were measured in diet-induced NASH mice. Adropin knock-out mice and palmitate treated primary hepatic cells were used to investigate the influence of adropin on liver injury. Our results show that serum adropin levels were decreased and negatively correlated with liver injury in NASH mice. Knockout of adropin significantly exacerbated hepatic steatosis, inflammatory responses and fibrosis in mice after either methionine-choline deficient diet (MCD) or western diet (WD) feeding. And the treatment with adropin bioactive peptides ameliorated NASH progression in mice. Adropin alleviated hepatocyte injury by upregulating the expression of Gclc, Gclm, and Gpx1 in a manner dependent on Nrf2 transcriptional activity and by increasing the glutathione (GSH) levels. And adropin significantly increased CBP expression and promoted its binding with Nrf2, which enhanced Nrf2 transcriptional activity. Furthermore, AAV8-mediated overexpression of hepatic Nrf2 expression functionally restored the liver injury induced by adropin-deficiency MCD-fed mice. These findings provide evidence that adropin activates Nrf2 signaling and plays a protective role in liver injury of NASH and therefore might represent a novel target for the prevention and treatment of NASH.
Keywords: GSH; Lipotoxicity; NASH; Nrf2; ROS.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Tiniakos D.G., Vos M.B., Brunt E.M. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu. Rev. Pathol. 2010;5:145–171. - PubMed
-
- Farrell G.C., Larter C.Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. - PubMed
-
- Brunt E.M. Pathology of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2010;7:195–203. - PubMed
-
- Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

