Chronic helminth infection does not impair immune response to malaria transmission blocking vaccine Pfs230D1-EPA/Alhydrogel® in mice
- PMID: 30685251
- PMCID: PMC6382667
- DOI: 10.1016/j.vaccine.2019.01.027
Chronic helminth infection does not impair immune response to malaria transmission blocking vaccine Pfs230D1-EPA/Alhydrogel® in mice
Abstract
Introduction: Malaria transmission blocking vaccines (TBV) are innovative approaches that aim to induce immunity in humans against Plasmodium during mosquito stage, neutralizing the capacity of the infected vectors to transmit malaria. Pfs230D1-EPA/Alhydrogel®, a promising protein-protein conjugate malaria TBV, is currently being tested in human clinical trials in areas where P. falciparum malaria is coendemic with helminth parasites. Helminths are complex metazoans that share the master capacity to downregulate the host immune response towards themselves and also to bystander antigens, including vaccines. However, it is not known whether the activity of a protein-based malaria TBV may be affected by a chronic helminth infection.
Methods: Using an experimental murine model for a chronic helminth infection (Heligmosomoides polygyrus bakeri - Hpb), we evaluated whether prior infection alters the activity of Pfs230D1-EPA/Alhydrogel® TBV in mice.
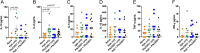
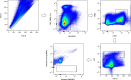
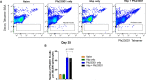
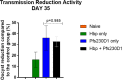
Results: After establishment of a chronic infection, characterized by a marked increase of parasite antigen-specific IgG1, IgA and IgE antibody responses, concomitant with an increase of systemic IL-10, IL-5 and IL-6 levels, the Hpb-infected mice were immunized with Pfs230D1-EPA/Alhydrogel® and the vaccine-specific immune response was compared with that in non-infected immunized mice. TBV immunizations induced an elevated vaccine specific-antibody response, however Pfs230D1 specific-IgG levels were similar between infected and uninfected mice at days 15, 25 and 35 post-vaccination. Absolute numbers of Pfs230D1-activated B cells generated in response to the vaccine were also similar among the vaccinated groups. Finally, vaccine activity assessed by reduction of oocyst number in P. falciparum infected mosquitoes was similar between Hpb-infected and immunized mice with non-infected immunized mice.
Conclusion: Pfs230D1-EPA/Alhydrogel® efficacy is not impaired by a chronic helminth infection in mice.
Keywords: H. polygyrus bakeri; Helminth intestinal infection; Immunoregulation; Malaria transmission blocking vaccine; P. falciparum; Pfs230.
Published by Elsevier Ltd.
Figures



Similar articles
-
Pfs230 yields higher malaria transmission-blocking vaccine activity than Pfs25 in humans but not mice.J Clin Invest. 2021 Apr 1;131(7):e146221. doi: 10.1172/JCI146221. J Clin Invest. 2021. PMID: 33561016 Free PMC article. Clinical Trial.
-
Malaria transmission-blocking vaccines Pfs230D1-EPA and Pfs25-EPA in Alhydrogel in healthy Malian adults; a phase 1, randomised, controlled trial.Lancet Infect Dis. 2023 Nov;23(11):1266-1279. doi: 10.1016/S1473-3099(23)00276-1. Epub 2023 Jul 24. Lancet Infect Dis. 2023. PMID: 37499679 Free PMC article. Clinical Trial.
-
The Pfs230 N-terminal fragment, Pfs230D1+: expression and characterization of a potential malaria transmission-blocking vaccine candidate.Malar J. 2019 Nov 8;18(1):356. doi: 10.1186/s12936-019-2989-2. Malar J. 2019. PMID: 31703583 Free PMC article.
-
Structural vaccinology of malaria transmission-blocking vaccines.Expert Rev Vaccines. 2021 Feb;20(2):199-214. doi: 10.1080/14760584.2021.1873135. Epub 2021 Jan 19. Expert Rev Vaccines. 2021. PMID: 33430656 Free PMC article. Review.
-
Malaria transmission blocking immunity and sexual stage vaccines for interrupting malaria transmission in Latin America.Mem Inst Oswaldo Cruz. 2011 Aug;106 Suppl 1(Suppl 1):202-11. doi: 10.1590/s0074-02762011000900025. Mem Inst Oswaldo Cruz. 2011. PMID: 21881775 Free PMC article. Review.
Cited by
-
Helminth Coinfections Modulate Disease Dynamics and Vaccination Success in the Era of Emerging Infectious Diseases.Vaccines (Basel). 2025 Apr 22;13(5):436. doi: 10.3390/vaccines13050436. Vaccines (Basel). 2025. PMID: 40432048 Free PMC article. Review.
-
A human monoclonal antibody blocks malaria transmission and defines a highly conserved neutralizing epitope on gametes.Nat Commun. 2021 Mar 19;12(1):1750. doi: 10.1038/s41467-021-21955-1. Nat Commun. 2021. PMID: 33741942 Free PMC article.
-
Immunomodulation of COVID-19 severity by helminth co-infection: Implications for COVID-19 vaccine efficacy.Immun Inflamm Dis. 2022 Mar;10(3):e573. doi: 10.1002/iid3.573. Epub 2021 Dec 3. Immun Inflamm Dis. 2022. PMID: 34861106 Free PMC article. Review.
-
Exploratory analysis of the effect of helminth infection on the immunogenicity and efficacy of the asexual blood-stage malaria vaccine candidate GMZ2.PLoS Negl Trop Dis. 2021 Jun 1;15(6):e0009361. doi: 10.1371/journal.pntd.0009361. eCollection 2021 Jun. PLoS Negl Trop Dis. 2021. PMID: 34061838 Free PMC article. Clinical Trial.
-
Antimalarial antibody repertoire defined by plasma IG proteomics and single B cell IG sequencing.JCI Insight. 2020 Nov 19;5(22):e143471. doi: 10.1172/jci.insight.143471. JCI Insight. 2020. PMID: 33048842 Free PMC article.
References
-
- Apiwattanakul N., Thomas P.G., Iverson A.R., McCullers J.A. Chronic helminth infections impair pneumococcal vaccine responses. Vaccine. 2014;32(42):5405–5410. - PubMed
-
- Ben-Smith A., Lammas D.A., Behnke J.M. The relative involvement of Th1 and Th2 associated immune responses in the expulsion of a primary infection of Heligmosomoides polygyrus in mice of differing response phenotype. J Helminthol. 2003;77(2):133–146. - PubMed
-
- Camberis M., Le Gros G., Urban J., Jr. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol. 2003 [Chapter 19: Unit 19 12] - PubMed
-
- Carter R. Transmission blocking malaria vaccines. Vaccine. 2001;19(17–19):2309–2314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

