Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort
- PMID: 30685685
- PMCID: PMC6506906
- DOI: 10.1016/j.ejca.2018.11.027
Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort
Abstract
Background: High-grade osteosarcoma is a primary malignant bone tumour mainly affecting children and young adults. The European and American Osteosarcoma Study (EURAMOS)-1 is a collaboration of four study groups aiming to improve outcomes of this rare disease by facilitating randomised controlled trials.
Methods: Patients eligible for EURAMOS-1 were aged ≤40 years with M0 or M1 skeletal high-grade osteosarcoma in which case complete surgical resection at all sites was deemed to be possible. A three-drug combination with methotrexate, doxorubicin and cisplatin was defined as standard chemotherapy, and between April 2005 and June 2011, 2260 patients were registered. We report survival outcomes and prognostic factors in the full cohort of registered patients.
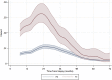
Results: For all registered patients at a median follow-up of 54 months (interquartile range: 38-73) from biopsy, 3-year and 5-year event-free survival were 59% (95% confidence interval [CI]: 57-61%) and 54% (95% CI: 52-56%), respectively. Multivariate analyses showed that the most adverse factors at diagnosis were pulmonary metastases (hazard ratio [HR] = 2.34, 95% CI: 1.95-2.81), non-pulmonary metastases (HR = 1.94, 95% CI: 1.38-2.73) or an axial skeleton tumour site (HR = 1.53, 95% CI: 1.10-2.13). The histological subtypes telangiectatic (HR = 0.52, 95% CI: 0.33-0.80) and unspecified conventional (HR = 0.67, 95% CI: 0.52-0.88) were associated with a favourable prognosis compared with chondroblastic subtype. The 3-year and 5-year overall survival from biopsy were 79% (95% CI: 77-81%) and 71% (95% CI: 68-73%), respectively. For patients with localised disease at presentation and in complete remission after surgery, having a poor histological response was associated with worse outcome after surgery (HR = 2.13, 95% CI: 1.76-2.58). In radically operated patients, there was no good evidence that axial tumour site was associated with worse outcome.
Conclusions: In conclusion, data from >2000 patients registered to EURAMOS-1 demonstrated survival rates in concordance with institution- or group-level osteosarcoma trials. Further efforts are required to drive improvements for patients who can be identified to be at higher risk of adverse outcome. This trial reaffirms known prognostic factors, and owing to the large numbers of patients registered, it sheds light on some additional factors to consider.
Keywords: Chemotherapy; Cohort; Osteosarcoma; Outcomes; Surgery.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Fletcher C.D. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology. 2014;64(1):2–11. - PubMed
-
- Marina N., Bielack S., Whelan J., Smeland S., Krailo M., Sydes M.R. International collaboration is feasible in trials for rare conditions: the EURAMOS experience. In: Jaffe N., Bruland O.S., Bielack S., editors. 1st ed. vol. 152. 2010. pp. 339–354. (Pediatric and adolescent osteosarcoma series: cancer treatment and research). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

