Imidazopyridines as Potent KDM5 Demethylase Inhibitors Promoting Reprogramming Efficiency of Human iPSCs
- PMID: 30685712
- PMCID: PMC6354736
- DOI: 10.1016/j.isci.2019.01.012
Imidazopyridines as Potent KDM5 Demethylase Inhibitors Promoting Reprogramming Efficiency of Human iPSCs
Abstract
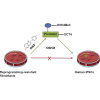
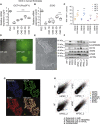
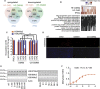
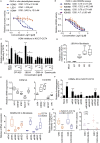
Pioneering human induced pluripotent stem cell (iPSC)-based pre-clinical studies have raised safety concerns and pinpointed the need for safer and more efficient approaches to generate and maintain patient-specific iPSCs. One approach is searching for compounds that influence pluripotent stem cell reprogramming using functional screens of known drugs. Our high-throughput screening of drug-like hits showed that imidazopyridines-analogs of zolpidem, a sedative-hypnotic drug-are able to improve reprogramming efficiency and facilitate reprogramming of resistant human primary fibroblasts. The lead compound (O4I3) showed a remarkable OCT4 induction, which at least in part is due to the inhibition of H3K4 demethylase (KDM5, also known as JARID1). Experiments demonstrated that KDM5A, but not its homolog KDM5B, serves as a reprogramming barrier by interfering with the enrichment of H3K4Me3 at the OCT4 promoter. Thus our results introduce a new class of KDM5 chemical inhibitors and provide further insight into the pluripotency-related properties of KDM5 family members.
Keywords: Biochemistry; Biological Sciences; Molecular Biology.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Histone demethylase KDM5 regulates cardiomyocyte maturation by promoting fatty acid oxidation, oxidative phosphorylation, and myofibrillar organization.bioRxiv [Preprint]. 2023 May 28:2023.04.11.535169. doi: 10.1101/2023.04.11.535169. bioRxiv. 2023. Update in: Cardiovasc Res. 2024 May 7;120(6):630-643. doi: 10.1093/cvr/cvae014. PMID: 37090524 Free PMC article. Updated. Preprint.
-
Inhibition of miRNA-212/132 improves the reprogramming of fibroblasts into induced pluripotent stem cells by de-repressing important epigenetic remodelling factors.Stem Cell Res. 2017 Apr;20:70-75. doi: 10.1016/j.scr.2017.03.003. Epub 2017 Mar 7. Stem Cell Res. 2017. PMID: 28314201
-
An Insight into DNA-free Reprogramming Approaches to Generate Integration-free Induced Pluripotent Stem Cells for Prospective Biomedical Applications.Stem Cell Rev Rep. 2019 Apr;15(2):286-313. doi: 10.1007/s12015-018-9861-6. Stem Cell Rev Rep. 2019. PMID: 30417242 Review.
-
Inducing goat pluripotent stem cells with four transcription factor mRNAs that activate endogenous promoters.BMC Biotechnol. 2017 Feb 13;17(1):11. doi: 10.1186/s12896-017-0336-7. BMC Biotechnol. 2017. PMID: 28193206 Free PMC article.
-
Current understanding and future perspectives of the roles of sirtuins in the reprogramming and differentiation of pluripotent stem cells.Exp Biol Med (Maywood). 2018 Mar;243(6):563-575. doi: 10.1177/1535370218759636. Exp Biol Med (Maywood). 2018. PMID: 29557214 Free PMC article. Review.
Cited by
-
Valproic Acid Thermally Destabilizes and Inhibits SpyCas9 Activity.Mol Ther. 2020 Dec 2;28(12):2635-2641. doi: 10.1016/j.ymthe.2020.08.014. Epub 2020 Aug 25. Mol Ther. 2020. PMID: 32882179 Free PMC article.
-
pVHL-mediated SMAD3 degradation suppresses TGF-β signaling.J Cell Biol. 2022 Jan 3;221(1):e202012097. doi: 10.1083/jcb.202012097. Epub 2021 Dec 3. J Cell Biol. 2022. PMID: 34860252 Free PMC article.
-
NHC-gold compounds mediate immune suppression through induction of AHR-TGFβ1 signalling in vitro and in scurfy mice.Commun Biol. 2020 Jan 3;3:10. doi: 10.1038/s42003-019-0716-8. eCollection 2020. Commun Biol. 2020. PMID: 31909202 Free PMC article.
-
A Novel Imidazopyridine Derivative Exerts Anticancer Activity by Inducing Mitochondrial Pathway-Mediated Apoptosis.Biomed Res Int. 2020 Aug 25;2020:4929053. doi: 10.1155/2020/4929053. eCollection 2020. Biomed Res Int. 2020. PMID: 32908894 Free PMC article.
-
A novel machine learning based approach for iPS progenitor cell identification.PLoS Comput Biol. 2019 Dec 26;15(12):e1007351. doi: 10.1371/journal.pcbi.1007351. eCollection 2019 Dec. PLoS Comput Biol. 2019. PMID: 31877128 Free PMC article.
References
-
- Atlasi Y., Stunnenberg H.G. The interplay of epigenetic marks during stem cell differentiation and development. Nat. Rev. Genet. 2017;18:643–658. - PubMed
-
- Baell J., Walters M.A. Chemical con artists foil drug discovery. Nature. 2014;513:481–483. - PubMed
-
- Buu-HoÏ N.P., Jacquignon P., Xuong N.D., Lavit D. 2-ARYLPYRROCOLINES AND 2-ARYLPYRIMIDAZOLES. J. Org. Chem. 1954;19:1370–1375.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

