Glucocorticoid-Induced Osteoporosis: New Insights into the Pathophysiology and Treatments
- PMID: 30685820
- PMCID: PMC6839409
- DOI: 10.1007/s11914-019-00498-x
Glucocorticoid-Induced Osteoporosis: New Insights into the Pathophysiology and Treatments
Abstract
Purpose of this review: The goal of the review is to provide an updated understanding of the pathophysiology of glucocorticoid-induced osteoporosis and treatment recommendations.
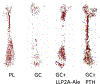
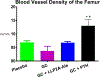
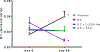
Recent findings: Glucocorticoids reduce osteoblast and osteocyte lifespan and activity and reduce the vascularity of the bone that together may explain the greater reductions in bone strength than those of bone mass. Treatments with parathyroid hormone fragments appear to reverse glucocorticoid-induced bone loss and fracture risk partially through maintaining bone vascularity and bone strength. This review identifies how glucocorticoid anti-osteogenic and vascular effects together may reduce bone strength. It also provides guidance to clinicians on rationale treatment for glucocorticoid-induced osteoporosis.
Keywords: Bone cells; Bone vascularity; Glucocorticoids; Osteonecrosis; Parathyroid hormone.
Conflict of interest statement
Compliance with Ethical Standards
Conflict of Interest
Nancy Lane reports having a patent (LLP2A-ale) issued.
Figures
References
-
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2004;15:323–328. 10.1007/s00198-003-1548-3. - DOI - PubMed
-
- Yao W, Cheng Z, Busse C, Pham A, Nakamura MC, Lane NE. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum. 2008;58:1674–1686. 10.1002/art.23954. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

