Enantioselective Allylation Using Allene, a Petroleum Cracking Byproduct
- PMID: 30685967
- PMCID: PMC6497157
- DOI: 10.1021/jacs.8b13907
Enantioselective Allylation Using Allene, a Petroleum Cracking Byproduct
Abstract
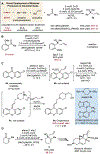
Allene (C3H4) gas is produced and separated on million-metric-ton scale per year during petroleum refining but is rarely employed in organic synthesis. Meanwhile, the addition of an allyl group (C3H5) to ketones is among the most common and prototypical reactions in synthetic chemistry. Herein, we report that the combination of allene gas with inexpensive and environmentally benign hydrosilanes, such as PMHS, can serve as a replacement for stoichiometric quantities of allylmetal reagents, which are required in most enantioselective ketone allylation reactions. This process is catalyzed by copper salts and commercially available ligands, operates without specialized equipment or pressurization, and tolerates a broad range of functional groups. Furthermore, the exceptional chemoselectivity of this catalyst system enables industrially relevant C3 hydrocarbon mixtures of allene with methylacetylene and propylene to be applied directly.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Gaich T; Baran PS Aiming For The Ideal Synthesis. J. Org. Chem 2010, 75, 4657–4673. - PubMed
- Anastas PT; Warner JC Green Chemistry: Theory and Practice; Oxford University Press: Oxford, U.K., 1998.
-
- Hu A; Guo J-J; Pan H; Zuo Z Selective Functionalization of Methane, Ethane, and Higher Alkanes by Cerium Photocatalysis. Science 2018, 361, 668–672. - PubMed
-
- Mo F; Dong. G. Regioselective Ketone α-Alkylation with Simple Olefins via Dual Activation. Science 2014, 345, 68–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

