Bi-allelic Mutations in ARMC2 Lead to Severe Astheno-Teratozoospermia Due to Sperm Flagellum Malformations in Humans and Mice
- PMID: 30686508
- PMCID: PMC6372258
- DOI: 10.1016/j.ajhg.2018.12.013
Bi-allelic Mutations in ARMC2 Lead to Severe Astheno-Teratozoospermia Due to Sperm Flagellum Malformations in Humans and Mice
Abstract
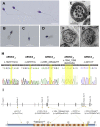
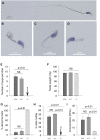
Male infertility is a major health concern. Among its different causes, multiple morphological abnormalities of the flagella (MMAF) induces asthenozoospermia and is one of the most severe forms of qualitative sperm defects. Sperm of affected men display short, coiled, absent, and/or irregular flagella. To date, six genes (DNAH1, CFAP43, CFAP44, CFAP69, FSIP2, and WDR66) have been found to be recurrently associated with MMAF, but more than half of the cases analyzed remain unresolved, suggesting that many yet-uncharacterized gene defects account for this phenotype. Here, whole-exome sequencing (WES) was performed on 168 infertile men who had a typical MMAF phenotype. Five unrelated affected individuals carried a homozygous deleterious mutation in ARMC2, a gene not previously linked to the MMAF phenotype. Using the CRISPR-Cas9 technique, we generated homozygous Armc2 mutant mice, which also presented an MMAF phenotype, thus confirming the involvement of ARMC2 in human MMAF. Immunostaining experiments in AMRC2-mutated individuals and mutant mice evidenced the absence of the axonemal central pair complex (CPC) proteins SPAG6 and SPEF2, whereas the other tested axonemal and peri-axonemal components were present, suggesting that ARMC2 is involved in CPC assembly and/or stability. Overall, we showed that bi-allelic mutations in ARMC2 cause male infertility in humans and mice by inducing a typical MMAF phenotype, indicating that this gene is necessary for sperm flagellum structure and assembly.
Keywords: Multiple morphological anomalies of the flagella (MMAF); cilia; flagella; infertility; spermatogenesis; spermatozoa.
Copyright © 2019 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Whole-exome sequencing identifies mutations in FSIP2 as a recurrent cause of multiple morphological abnormalities of the sperm flagella.Hum Reprod. 2018 Oct 1;33(10):1973-1984. doi: 10.1093/humrep/dey264. Hum Reprod. 2018. PMID: 30137358
-
Patients with multiple morphological abnormalities of the sperm flagella due to DNAH1 mutations have a good prognosis following intracytoplasmic sperm injection.Hum Reprod. 2016 Jun;31(6):1164-72. doi: 10.1093/humrep/dew083. Epub 2016 Apr 19. Hum Reprod. 2016. PMID: 27094479
-
Biallelic Mutations in CFAP43 and CFAP44 Cause Male Infertility with Multiple Morphological Abnormalities of the Sperm Flagella.Am J Hum Genet. 2017 Jun 1;100(6):854-864. doi: 10.1016/j.ajhg.2017.04.012. Epub 2017 May 25. Am J Hum Genet. 2017. PMID: 28552195 Free PMC article.
-
Insight on multiple morphological abnormalities of sperm flagella in male infertility: what is new?Asian J Androl. 2020 May-Jun;22(3):236-245. doi: 10.4103/aja.aja_53_19. Asian J Androl. 2020. PMID: 31210147 Free PMC article. Review.
-
The genetic architecture of morphological abnormalities of the sperm tail.Hum Genet. 2021 Jan;140(1):21-42. doi: 10.1007/s00439-020-02113-x. Epub 2020 Jan 16. Hum Genet. 2021. PMID: 31950240 Review.
Cited by
-
Novel biallelic loss-of-function mutations in CFAP43 cause multiple morphological abnormalities of the sperm flagellum in Pakistani families.Asian J Androl. 2021 Nov-Dec;23(6):627-632. doi: 10.4103/aja.aja_26_21. Asian J Androl. 2021. PMID: 34100391 Free PMC article.
-
Novel DNAH1 Mutation Loci Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Literature Review.World J Mens Health. 2022 Oct;40(4):551-560. doi: 10.5534/wjmh.210119. Epub 2022 Jan 25. World J Mens Health. 2022. PMID: 35118838 Free PMC article. Review.
-
The Emerging Role of Sperm-Associated Antigen 6 Gene in the Microtubule Function of Cells and Cancer.Mol Ther Oncolytics. 2019 Sep 10;15:101-107. doi: 10.1016/j.omto.2019.08.011. eCollection 2019 Dec 20. Mol Ther Oncolytics. 2019. PMID: 31660426 Free PMC article. Review.
-
Bi-allelic Mutations in TTC21A Induce Asthenoteratospermia in Humans and Mice.Am J Hum Genet. 2019 Apr 4;104(4):738-748. doi: 10.1016/j.ajhg.2019.02.020. Epub 2019 Mar 28. Am J Hum Genet. 2019. PMID: 30929735 Free PMC article.
-
How exome sequencing improves the diagnostics and management of men with non-syndromic infertility.Andrology. 2025 Jul;13(5):1011-1024. doi: 10.1111/andr.13728. Epub 2024 Aug 9. Andrology. 2025. PMID: 39120565 Free PMC article. Review.
References
-
- Krausz C., Riera-Escamilla A. Genetics of male infertility. Nat. Rev. Urol. 2018;15:369–384. - PubMed
-
- Coutton C., Escoffier J., Martinez G., Arnoult C., Ray P.F. Teratozoospermia: spotlight on the main genetic actors in the human. Hum. Reprod. Update. 2015;21:455–485. - PubMed
-
- Ray P.F., Toure A., Metzler-Guillemain C., Mitchell M.J., Arnoult C., Coutton C. Genetic abnormalities leading to qualitative defects of sperm morphology or function. Clin. Genet. 2017;91:217–232. - PubMed

