Adaptive F-Actin Polymerization and Localized ATP Production Drive Basement Membrane Invasion in the Absence of MMPs
- PMID: 30686527
- PMCID: PMC6372315
- DOI: 10.1016/j.devcel.2018.12.018
Adaptive F-Actin Polymerization and Localized ATP Production Drive Basement Membrane Invasion in the Absence of MMPs
Abstract
Matrix metalloproteinases (MMPs) are associated with decreased patient prognosis but have failed as anti-invasive drug targets despite promoting cancer cell invasion. Through time-lapse imaging, optical highlighting, and combined genetic removal of the five MMPs expressed during anchor cell (AC) invasion in C. elegans, we find that MMPs hasten invasion by degrading basement membrane (BM). Though irregular and delayed, AC invasion persists in MMP- animals via adaptive enrichment of the Arp2/3 complex at the invasive cell membrane, which drives formation of an F-actin-rich protrusion that physically breaches and displaces BM. Using a large-scale RNAi synergistic screen and a genetically encoded ATP FRET sensor, we discover that mitochondria enrich within the protrusion and provide localized ATP that fuels F-actin network growth. Thus, without MMPs, an invasive cell can alter its BM-breaching tactics, suggesting that targeting adaptive mechanisms will be necessary to mitigate BM invasion in human pathologies.
Keywords: ATP transport; actin dynamics; basement membrane; invasion; live imaging; matrix metalloproteinase; mitochondria.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATIONS OF INTEREST
The authors declare no competing interests.
Figures
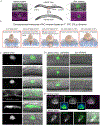
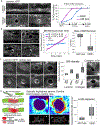

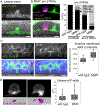
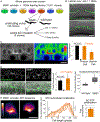
Comment in
-
Forcing through barriers.Nat Rev Mol Cell Biol. 2019 Mar;20(3):136. doi: 10.1038/s41580-019-0104-8. Nat Rev Mol Cell Biol. 2019. PMID: 30710120 No abstract available.
-
Invasion by Force: The C. elegans Anchor Cell Leads the Way.Dev Cell. 2019 Feb 11;48(3):287-288. doi: 10.1016/j.devcel.2019.01.024. Dev Cell. 2019. PMID: 30753832
References
-
- Altincicek B, Fischer M, Fischer M, Lüersen K, Boll M, Wenzel U, and Vilcinskas A (2010). Role of matrix metalloproteinase ZMP-2 in pathogen resistance and development in Caenorhabditis elegans. Dev Comp Immunol 34, 1160–1169. - PubMed
-
- Arismendi-Morillo G, Hoa NT, Ge L, and Jadus MR (2012). Mitochondrial network in glioma’s invadopodia displays an activated state both in situ and in vitro: potential functional implications. Ultrastruct Pathol 36, 409–414. - PubMed
-
- Boone C, Bussey H, and Andrews BJ (2007). Exploring genetic interactions and networks with yeast. Nat Rev Genet 8, 437–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

