A Phase III Study to Evaluate the Immunogenicity and Safety of GC1107 (Adult Tetanus Diphtheria Vaccine) in Healthy Adults
- PMID: 30686952
- PMCID: PMC6345633
- DOI: 10.3346/jkms.2019.34.e31
A Phase III Study to Evaluate the Immunogenicity and Safety of GC1107 (Adult Tetanus Diphtheria Vaccine) in Healthy Adults
Abstract
Background: This study was conducted to assess the immunogenicity and safety of GC1107 (adult tetanus diphtheria [Td] vaccine). The primary goal was to evaluate the non-inferiority of the immunogenicity of GC1107 compared to the control vaccine. Additionally, the safety profiles of GC1107 and the control vaccine were compared.
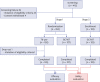
Methods: The subjects were adults ≥ 18 years old who were not injected with Td or adult tetanus-diphtheria-pertussis (TdaP) vaccine within the recent 5 years. A total of 253 subjects were enrolled and randomized to either the GC1107 group or the control group. For immunogenicity assessment, blood samples were collected at baseline and 28 days after vaccination and antibody titer of diphtheria and tetanus were assessed.
Results: The seroprotection rates of diphtheria and tetanus were 89.76% and 91.34%, respectively, in the GC1107 group, and 87.80% and 86.99% in the control group. The geometric mean titer (GMT) of the anti-diphtheria antibody increased after vaccination in both groups, showing no significant difference between the groups (P = 0.139). The anti-tetanus GMTs after vaccination also showed comparable increases in both groups, and showed no significant difference (P = 0.860). In the safety evaluation, solicited local adverse reactions occurred in 81.2% of the subjects in the GC1107 group and in 86.4% of the subjects in the control group. Solicited systemic adverse events occurred in 33.2% of the subjects in the GC1107 group and in 47.2% of the subjects in the control group, which did not reach statistical significance.
Conclusion: This phase III study demonstrated non-inferiority in immunogenicity and comparable safety of GC1107 compared with the control Td vaccine.
Trial registration: ClinicalTrials.gov Identifier: NCT02361866.
Keywords: Immunogenicity; Safety; Td; Vaccine.
Conflict of interest statement
Disclosure: The authors have no potential conflicts of interest to disclose.
Figures
Comment in
-
Vaccines of Our Own.J Korean Med Sci. 2019 Jan 21;34(4):e33. doi: 10.3346/jkms.2019.34.e33. eCollection 2019 Jan 28. J Korean Med Sci. 2019. PMID: 30686954 Free PMC article. No abstract available.
References
-
- Tiwari TS, Wharton M. Diphtheria toxoid. In: Plotkin SA, Orsentein WA, Offit PA, Edwards KM, editors. Plotkin's Vaccines. 7th ed. Philadelphia, PA: Elsevier; 2018. pp. 261–275.
-
- Roper MH, Wassilak SG, Scobie HM, Ridpath AD, Orenstein WA. Tetanus toxoid. In: Plotkin SA, Orsentein WA, Offit PA, Edwards KM, editors. Plotkin's Vaccines. 7th ed. Philadelphia, PA: Elsevier; 2018. pp. 1052–1080.
-
- Lee SY, Kim JS, Ahn JH, Choi JH, Ma SH, Park JS, et al. Immunoassay of diphtheria and tetanus according to ages. Infect Chemother. 2012;44(2):62–66.
-
- Hamborsky J, Kroeger A, Wolfe S. Epidemiology and Prevention of Vaccine-preventable Diseases. 13th ed. Washington, D.C.: Centers for Disease Control and Prevention; 2015.
-
- Hardy IR, Dittmann S, Sutter RW. Current situation and control strategies for resurgence of diphtheria in newly independent states of the former Soviet Union. Lancet. 1996;347(9017):1739–1744. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical