Tau and mTOR: The Hotspots for Multifarious Diseases in Alzheimer's Development
- PMID: 30686983
- PMCID: PMC6335350
- DOI: 10.3389/fnins.2018.01017
Tau and mTOR: The Hotspots for Multifarious Diseases in Alzheimer's Development
Abstract
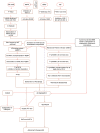
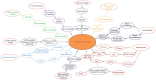
The hyperphosphorylation of tau protein and the overexpression of mTOR are considered to be the driving force behind Aβ plaques and Neurofibrillay Tangles (NFT's), hallmarks of Alzheimer's disease (AD). It is now evident that miscellaneous diseases such as Diabetes, Autoimmune diseases, Cancer, etc. are correlated with AD. Therefore, we reviewed the literature on the causes of AD and investigated the association of tau and mTOR with other diseases. We have discussed the role of insulin deficiency in diabetes, activated microglial cells, and dysfunction of blood-brain barrier (BBB) in Autoimmune diseases, Presenilin 1 in skin cancer, increased reactive species in mitochondrial dysfunction and deregulated Cyclins/CDKs in promoting AD pathogenesis. We have also discussed the possible therapeutics for AD such as GSK3 inactivation therapy, Rechaperoning therapy, Immunotherapy, Hormonal therapy, Metal chelators, Cell cycle therapy, γ-secretase modulators, and Cholinesterase and BACE 1-inhibitors which are thought to serve a major role in combating pathological changes coupled with AD. Recent research about the relationship between mTOR and aging and hepatic Aβ degradation offers possible targets to effectively target AD. Future prospects of AD aims at developing novel drugs and modulators that can potentially improve cell to cell signaling, prevent Aβ plaques formation, promote better release of neurotransmitters and prevent hyperphosphorylation of tau.
Keywords: Alzheimer's disease (AD); Aβ modulators; Aβ plaques; NFT's; hyperphosphorylation; mTOR; tau.
Figures
References
-
- Adolfsson O., Pihlgren M., Toni N., Varisco Y., Buccarello A. L., Antoniello K., et al. (2012). An effector-reduced anti-β-amyloid (Aβ) antibody with unique aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J. Neurosci. 32, 9677–9689. 10.1523/JNEUROSCI.4742-11.2012 - DOI - PMC - PubMed
-
- Baker L. D., Cross D. J., Minoshima S., Belongia D., Watson G. S., Craft S. (2011). Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol. 68, 51–57. 10.1001/archneurol.2010.225 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

