Disruption of Otoferlin Alters the Mode of Exocytosis at the Mouse Inner Hair Cell Ribbon Synapse
- PMID: 30687007
- PMCID: PMC6338019
- DOI: 10.3389/fnmol.2018.00492
Disruption of Otoferlin Alters the Mode of Exocytosis at the Mouse Inner Hair Cell Ribbon Synapse
Abstract
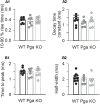
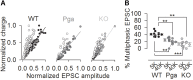
Sound encoding relies on Ca2+-mediated exocytosis at the ribbon synapse between cochlear inner hair cells (IHCs) and type I spiral ganglion neurons (SGNs). Otoferlin, a multi-C2 domain protein, is proposed to regulate Ca2+-triggered exocytosis at this synapse, but the precise mechanisms of otoferlin function remain to be elucidated. Here, performing whole-cell voltage-clamp recordings of excitatory postsynaptic currents (EPSCs) from SGNs in otoferlin mutant mice, we investigated the impact of Otof disruption at individual synapses with single release event resolution. Otof deletion decreased the spontaneous release rate and abolished the stimulus-secretion coupling. This was evident from failure of potassium-induced IHC depolarization to stimulate release and supports the proposed role of otoferlin in Ca2+ sensing for fusion. A missense mutation in the Otof gene (pachanga), in which otoferlin level at the IHC plasma membrane was lowered without changing its Ca2+ binding, also reduced the spontaneous release rate but spared the stimulus-secretion coupling. The slowed stimulated release rate supports the hypothesis that a sufficient abundance of otoferlin at the plasma membrane is crucial for the vesicle supply. Large-sized monophasic EPSCs remained present upon Otof deletion despite the drastic reduction of the rate of exocytosis. However, EPSC amplitude, on average, was modestly decreased. Moreover, a reduced contribution of multiphasic EPSC was observed in both Otof mutants. We argue that the presence of large monophasic EPSCs despite the exocytic defect upon Otof deletion supports the uniquantal hypothesis of transmitter release at the IHC ribbon synapse. Based upon the reduced contribution of multiphasic EPSC, we propose a role of otoferlin in regulating the mode of exocytosis in IHCs.
Keywords: EPSC; auditory; calcium; cochlea; hair cell; otoferlin; ribbon synapse; spiral ganglion neuron.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

