Neuropeptides Exert Neuroprotective Effects in Alzheimer's Disease
- PMID: 30687008
- PMCID: PMC6336706
- DOI: 10.3389/fnmol.2018.00493
Neuropeptides Exert Neuroprotective Effects in Alzheimer's Disease
Abstract
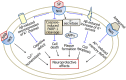
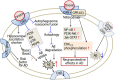
Alzheimer's disease (AD) is an age-related neurodegenerative disorder characterized by cognitive deficits and neuronal loss. Deposition of beta-amyloid peptide (Aβ) causes neurotoxicity through the formation of plaques in brains of Alzheimer's disease. Numerous studies have indicated that the neuropeptides including ghrelin, neurotensin, pituitary adenylate cyclase-activating polypeptide (PACAP), neuropeptide Y, substance P and orexin are closely related to the pathophysiology of Alzheimer's disease. The levels of neuropeptides and their receptors change in Alzheimer's disease. These neuropeptides exert neuroprotective roles mainly through preventing Aβ accumulation, increasing neuronal glucose transport, increasing the production of neurotrophins, inhibiting endoplasmic reticulum stress and autophagy, modulating potassium channel activity and hippocampal long-term potentiation. Therefore, the neuropeptides may function as potential drug targets in the prevention and cure of Alzheimer's disease.
Keywords: Alzheimer's disease; ghrelin; neuropeptide; neuropeptide Y; neurotensin; orexin; pituitary adenylate cyclase-activating polypeptide; substance P.
Figures
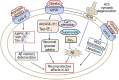
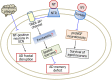
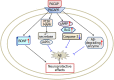
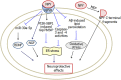
Similar articles
-
The Emerging Role of Neuropeptides in Parkinson's Disease.Front Aging Neurosci. 2021 Mar 8;13:646726. doi: 10.3389/fnagi.2021.646726. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33762925 Free PMC article. Review.
-
The neuropeptide PACAP attenuates beta-amyloid (1-42)-induced toxicity in PC12 cells.Peptides. 2002 Aug;23(8):1471-8. doi: 10.1016/s0196-9781(02)00085-2. Peptides. 2002. PMID: 12182949
-
Role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the action of ginsenoside Rh2 against beta-amyloid-induced inhibition of rat brain astrocytes.Neurosci Lett. 2008 Mar 21;434(1):1-5. doi: 10.1016/j.neulet.2007.12.032. Epub 2007 Dec 23. Neurosci Lett. 2008. PMID: 18313848
-
Protective Effects of Pituitary Adenylate Cyclase-Activating Polypeptide and Vasoactive Intestinal Peptide Against Cognitive Decline in Neurodegenerative Diseases.Front Cell Neurosci. 2020 Jul 17;14:221. doi: 10.3389/fncel.2020.00221. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32765225 Free PMC article.
-
Pituitary adenylate cyclase-activating polypeptides, PACAP-38 and PACAP-27, regulation of sympathetic neuron catecholamine, and neuropeptide Y expression through activation of type I PACAP/VIP receptor isoforms.Ann N Y Acad Sci. 1996 Dec 26;805:204-16; discussion 217-8. doi: 10.1111/j.1749-6632.1996.tb17484.x. Ann N Y Acad Sci. 1996. PMID: 8993404 Review.
Cited by
-
Polyamine Dysregulation and Nucleolar Disruption in Alzheimer's Disease.J Alzheimers Dis. 2024;98(3):837-857. doi: 10.3233/JAD-231184. J Alzheimers Dis. 2024. PMID: 38489184 Free PMC article.
-
Alzheimer's Disease Mouse as a Model of Testis Degeneration.Int J Mol Sci. 2020 Aug 10;21(16):5726. doi: 10.3390/ijms21165726. Int J Mol Sci. 2020. PMID: 32785075 Free PMC article.
-
Roles of Neuropeptide Y in Neurodegenerative and Neuroimmune Diseases.Front Neurosci. 2019 Aug 20;13:869. doi: 10.3389/fnins.2019.00869. eCollection 2019. Front Neurosci. 2019. PMID: 31481869 Free PMC article. Review.
-
Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders.Metabolites. 2022 Jun 18;12(6):562. doi: 10.3390/metabo12060562. Metabolites. 2022. PMID: 35736494 Free PMC article.
-
Potentials of Neuropeptides as Therapeutic Agents for Neurological Diseases.Biomedicines. 2022 Feb 1;10(2):343. doi: 10.3390/biomedicines10020343. Biomedicines. 2022. PMID: 35203552 Free PMC article. Review.
References
-
- Ahmed M. M., Hoshino H., Chikuma T., Yamada M., Kato T. (2004). Effect of memantine on the levels of glial cells, neuropeptides, and peptide-degrading enzymes in rat brain regions of ibotenic acid-treated Alzheimer's disease model. Neuroscience 126, 639–649. 10.1016/j.neuroscience.2004.04.024 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

