Locus Coeruleus Optogenetic Light Activation Induces Long-Term Potentiation of Perforant Path Population Spike Amplitude in Rat Dentate Gyrus
- PMID: 30687027
- PMCID: PMC6333706
- DOI: 10.3389/fnsys.2018.00067
Locus Coeruleus Optogenetic Light Activation Induces Long-Term Potentiation of Perforant Path Population Spike Amplitude in Rat Dentate Gyrus
Abstract
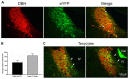
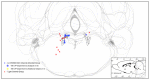
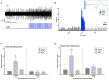
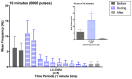
Norepinephrine (NE) in dentate gyrus (DG) produces NE-dependent long-term potentiation (NE-LTP) of the perforant path-evoked potential population spike both in vitro and in vivo. Chemical activators infused near locus coeruleus (LC), the source of DG NE, produce a NE-LTP that is associative, i.e., requires concurrent pairing with perforant path (PP) input. Here, we ask if LC optogenetic stimulation that allows us to activate only LC neurons can induce NE-LTP in DG. We use an adeno-associated viral vector containing a depolarizing channel (AAV8-Ef1a-DIO-eChR2(h134r)-EYFP-WPRE) infused stereotaxically into the LC of TH:Cre rats to produce light-sensitive LC neurons. A co-localization of ~62% in LC neurons was observed for these channels. Under urethane anesthesia, we demonstrated that 5-10 s 10 Hz trains of 30 ms light pulses in LC reliably activated neurons near an LC optoprobe. Ten minutes of the same train paired with 0.1 Hz PP electrical stimulation produced a delayed NE-LTP of population spike amplitude, but not EPSP slope. A leftward shift in the population spike input/output curve at the end of the experiment was also consistent with long-term population spike potentiation. LC neuron activity during the 10 min light train was unexpectedly transient. Increased LC neuronal firing was seen only for the first 2 min of the light train. NE-LTP was more delayed and less robust than reported with LC chemo-activation. Previous estimates of LC axonal conduction times suggest acute release of NE occurs 40-70 ms after an LC neuron action potential. We used single LC light pulses to examine acute effects of NE release and found potentiated population spike amplitude when a light pulse in LC occurred 40-50 ms, but not 20-30 ms, prior to a PP pulse, consistent with conduction estimates. These effects of LC optogenetic activation reinforce evidence for a continuum of NE potentiation effects in DG. The single pulse effects mirror an earlier report using LC electrical stimulation. These acute effects support an attentional role of LC activation. The LTP of PP responses induced by optogenetic LC activation is consistent with the role of LC in long-term learning and memory.
Keywords: dentate gyrus; hippocampus; locus coeruleus; long-term potentiation; norepinephrine; optogenetic; perforant path; short-term potentiation.
Figures



Similar articles
-
Medial and lateral perforant path evoked potentials are selectively modulated by pairing with glutamatergic activation of locus coeruleus in the dentate gyrus of the anesthetized rat.Hippocampus. 2012 Mar;22(3):501-9. doi: 10.1002/hipo.20916. Epub 2011 Jan 14. Hippocampus. 2012. PMID: 21240916
-
An associativity requirement for locus coeruleus-induced long-term potentiation in the dentate gyrus of the urethane-anesthetized rat.Exp Brain Res. 2010 Jan;200(2):151-9. doi: 10.1007/s00221-009-1955-6. Epub 2009 Jul 31. Exp Brain Res. 2010. PMID: 19644680
-
Idazoxan increases perforant path-evoked EPSP slope paired pulse inhibition and reduces perforant path-evoked population spike paired pulse facilitation in rat dentate gyrus.Brain Res. 2006 Feb 9;1072(1):36-45. doi: 10.1016/j.brainres.2005.12.020. Epub 2006 Jan 19. Brain Res. 2006. PMID: 16426582
-
Noradrenergic and locus coeruleus modulation of the perforant path-evoked potential in rat dentate gyrus supports a role for the locus coeruleus in attentional and memorial processes.Prog Brain Res. 1991;88:307-21. doi: 10.1016/s0079-6123(08)63818-2. Prog Brain Res. 1991. PMID: 1687619 Review.
-
Long-term potentiation: studies in the hippocampal slice.J Neurosci Methods. 1989 May;28(1-2):109-24. doi: 10.1016/0165-0270(89)90016-2. J Neurosci Methods. 1989. PMID: 2542698 Review.
Cited by
-
Spatial contextual recognition memory updating is modulated by dopamine release in the dorsal hippocampus from the locus coeruleus.Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2208254119. doi: 10.1073/pnas.2208254119. Epub 2022 Nov 29. Proc Natl Acad Sci U S A. 2022. PMID: 36442129 Free PMC article.
-
The locus coeruleus contributes to the anorectic, nausea, and autonomic physiological effects of glucagon-like peptide-1.Sci Adv. 2023 Sep 22;9(38):eadh0980. doi: 10.1126/sciadv.adh0980. Epub 2023 Sep 20. Sci Adv. 2023. PMID: 37729419 Free PMC article.
-
Frequency Specific Optogenetic Stimulation of the Locus Coeruleus Induces Task-Relevant Plasticity in the Motor Cortex.J Neurosci. 2024 Feb 14;44(7):e1528232023. doi: 10.1523/JNEUROSCI.1528-23.2023. J Neurosci. 2024. PMID: 38124020 Free PMC article.
-
Bilateral Optogenetic Stimulation of the Olfactory Bulb of OMP-ChIEF Mice.Methods Mol Biol. 2025;2915:189-200. doi: 10.1007/978-1-0716-4466-9_13. Methods Mol Biol. 2025. PMID: 40249493
-
Locus Coeruleus Optogenetic Modulation: Lessons Learned from Temporal Patterns.Brain Sci. 2021 Dec 9;11(12):1624. doi: 10.3390/brainsci11121624. Brain Sci. 2021. PMID: 34942924 Free PMC article.
References
-
- Assaf S. Y., Mason S. T., Miller J. J. (1979). Noradrenergic modulation transmission between the entorhinal cortex and the dentate gyrus of the rat [proceedings]. J. Physiol. 292:52P. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

