The Expression of Transcription Factors Mecp2 and CREB Is Modulated in Inflammatory Pelvic Pain
- PMID: 30687029
- PMCID: PMC6336837
- DOI: 10.3389/fnsys.2018.00069
The Expression of Transcription Factors Mecp2 and CREB Is Modulated in Inflammatory Pelvic Pain
Abstract
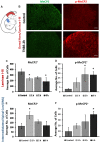
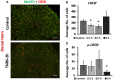
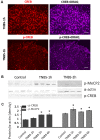
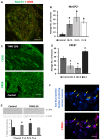
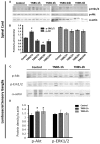
Early activation of transcription factors is one of the epigenetic mechanisms contributing to the induction and maintenance of chronic pain states. Previous studies identified the changes in a number of nociception-related genes, such as calcitonin gene-related peptide (CGRP), substance P (SP), and brain-derived neurotropic factor (BDNF) in the pelvic organs after transient colonic inflammation. The gene and protein expression of these neuropeptides could be modulated by transcription factors Methyl-CpG-binding protein 2 (Mecp2) and cAMP response element-binding protein (CREB). In this study, we aimed to evaluate time-dependent changes in the expression levels of Mecp2 and CREB in the lumbosacral (LS) spinal cord and sensory ganglia after inflammation-induced pelvic pain in rat. Adult Sprague-Dawley rats were treated with 2,4,6-trinitrobenzenesulfonic acid (TNBS) to induce transient colonic inflammation. LS (L6-S2) spinal cord segments and respective dorsal root ganglias (DRGs) were isolated from control and experimental animals at 1, 2, 6, 24 h and 3 days post-TNBS treatment. Immunohistochemical (IHC) labeling and Western blotting experiments were performed to assess the expression of Mecp2, CREB and their phosphorylated forms. Total Mecp2 expression, but not phosphorylated p-Mecp2 (pS421Mecp2) expression was detected in the cells of the spinal dorsal horn under control conditions. Colonic inflammation triggered a significant decrease in the number of Mecp2-expressing neurons in parallel with elevated numbers of pS421Mecp2-expressing cells at 2 h and 6 h post-TNBS. The majority of Mecp2-positive cells (80 ± 6%) co-expressed CREB. TNBS treatment caused a transient up-regulation of CREB-expressing cells at 1 h post-TNBS only. The number of cells expressing phosphorylated CREB (pS133CREB) did not change at 1 h and 2 h post-TNBS, but was down-regulated by three folds at 6 h post-TNBS. Analysis of DRG sections revealed that the number of Mecp2-positive neurons was up-regulated by TNBS treatment, reaching three-fold increase at 2 h post-TNBS, and eight-fold increase at 6 h post-TNBS (p ≤ 0.05 to control). These data showed early changes in Mecp2 and CREB expression in the dorsal horn of the spinal cord and sensory ganglia after colonic inflammation, suggesting a possible contribution Mecp2 and CREB signaling in the development of visceral hyperalgesia and pelvic pain following peripheral inflammation.
Keywords: CREB; Mecp2; TNBS; dorsal root ganglia; inflammation; spinal cord; visceral pain.
Figures
Similar articles
-
Experimental colitis triggers the release of substance P and calcitonin gene-related peptide in the urinary bladder via TRPV1 signaling pathways.Exp Neurol. 2010 Oct;225(2):262-73. doi: 10.1016/j.expneurol.2010.05.012. Epub 2010 May 23. Exp Neurol. 2010. PMID: 20501335 Free PMC article.
-
Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: an in vitro and in vivo study.Eur J Neurosci. 2001 Oct;14(7):1091-104. doi: 10.1046/j.0953-816x.2001.01731.x. Eur J Neurosci. 2001. PMID: 11683901
-
Axotomy of tributaries of the pelvic and pudendal nerves induces changes in the neurochemistry of mouse dorsal root ganglion neurons and the spinal cord.Brain Struct Funct. 2016 May;221(4):1985-2004. doi: 10.1007/s00429-015-1019-6. Epub 2015 Mar 7. Brain Struct Funct. 2016. PMID: 25749859 Free PMC article.
-
Changes of the neuropeptides content and gene expression in spinal cord and dorsal root ganglion after noxious colorectal distension.Regul Pept. 2005 Nov;131(1-3):66-73. doi: 10.1016/j.regpep.2005.06.008. Regul Pept. 2005. PMID: 16084604
-
The peptide content of colonic afferents decreases following colonic inflammation.Peptides. 1999;20(2):267-73. doi: 10.1016/s0196-9781(98)00157-0. Peptides. 1999. PMID: 10422883
Cited by
-
Activation of CB1R alleviates central sensitization by regulating HCN2-pNR2B signaling in a chronic migraine rat model.J Headache Pain. 2023 Apr 21;24(1):44. doi: 10.1186/s10194-023-01580-7. J Headache Pain. 2023. PMID: 37085778 Free PMC article.
-
Dysfunction of inhibitory interneurons contributes to synaptic plasticity via GABABR-pNR2B signaling in a chronic migraine rat model.Front Mol Neurosci. 2023 May 26;16:1142072. doi: 10.3389/fnmol.2023.1142072. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37324588 Free PMC article.
-
Rett syndrome: advances in Understanding MeCP2 function, potential gene therapies, and public health implications.Mol Biol Rep. 2025 Jul 8;52(1):687. doi: 10.1007/s11033-025-10802-x. Mol Biol Rep. 2025. PMID: 40627220 Review.
-
The changes of nociception and the signal molecules expression in the dorsal root ganglia and the spinal cord after cold water swimming stress in mice.Korean J Physiol Pharmacol. 2021 May 1;25(3):207-216. doi: 10.4196/kjpp.2021.25.3.207. Korean J Physiol Pharmacol. 2021. PMID: 33859061 Free PMC article.
-
MeCP2 Epigenetic Silencing of Oprm1 Gene in Primary Sensory Neurons Under Neuropathic Pain Conditions.Front Neurosci. 2021 Nov 5;15:743207. doi: 10.3389/fnins.2021.743207. eCollection 2021. Front Neurosci. 2021. PMID: 34803588 Free PMC article.
References
-
- Asfaw T. S., Hypolite J., Northington G. M., Arya L. A., Wein A. J., Malykhina A. P. (2011). Acute colonic inflammation triggers detrusor instability via activation of TRPV1 receptors in a rat model of pelvic organ cross-sensitization. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1392–R1400. 10.1152/ajpregu.00804.2010 - DOI - PMC - PubMed
-
- Bhattacherjee A., Mu Y., Winter M. K., Knapp J. R., Eggimann L. S., Gunewardena S. S., et al. . (2017). Neuronal cytoskeletal gene dysregulation and mechanical hypersensitivity in a rat model of Rett syndrome. Proc. Natl. Acad. Sci. U S A 114, E6952–E6961. 10.1073/pnas.1618210114 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

