Experience-Dependent Effects of Muscimol-Induced Hippocampal Excitation on Mnemonic Discrimination
- PMID: 30687032
- PMCID: PMC6335355
- DOI: 10.3389/fnsys.2018.00072
Experience-Dependent Effects of Muscimol-Induced Hippocampal Excitation on Mnemonic Discrimination
Abstract
Memory requires similar episodes with overlapping features to be represented distinctly, a process that is disrupted in many clinical conditions as well as normal aging. Data from humans have linked this ability to activity in hippocampal CA3 and dentate gyrus (DG). While animal models have shown the perirhinal cortex is critical for disambiguating similar stimuli, hippocampal activity has not been causally linked to discrimination abilities. The goal of the current study was to determine how disrupting CA3/DG activity would impact performance on a rodent mnemonic discrimination task. Rats were surgically implanted with bilateral guide cannulae targeting dorsal CA3/DG. In Experiment 1, the effect of intra-hippocampal muscimol on target-lure discrimination was assessed within subjects in randomized blocks. Muscimol initially impaired discrimination across all levels of target-lure similarity, but performance improved on subsequent test blocks irrespective of stimulus similarity and infusion condition. To clarify these results, Experiment 2 examined whether prior experience with objects influenced the effect of muscimol on target-lure discrimination. Rats that received vehicle infusions in a first test block, followed by muscimol in a second block, did not show discrimination impairments for target-lure pairs of any similarity. In contrast, rats that received muscimol infusions in the first test block were impaired across all levels of target-lure similarity. Following discrimination tests, rats from Experiment 2 were trained on a spatial alternation task. Muscimol infusions increased the number of spatial errors made, relative to vehicle infusions, confirming that muscimol remained effective in disrupting behavioral performance. At the conclusion of behavioral experiments, fluorescence in situ hybridization for the immediate-early genes Arc and Homer1a was used to determine the proportion of neurons active following muscimol infusion. Contrary to expectations, muscimol increased neural activity in DG. An additional experiment was carried out to quantify neural activity in naïve rats that received an intra-hippocampal infusion of vehicle or muscimol. Results confirmed that muscimol led to DG excitation, likely through its actions on interneuron populations in hilar and molecular layers of DG and consequent disinhibition of principal cells. Taken together, our results suggest disruption of coordinated neural activity across the hippocampus impairs mnemonic discrimination when lure stimuli are novel.
Keywords: CA3; aging; dentate gyrus; epilepsy; object recognition; perirhinal cortex.
Figures
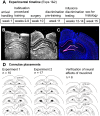

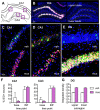



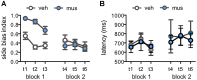
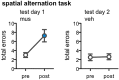
Similar articles
-
Rodent mnemonic similarity task performance requires the prefrontal cortex.Hippocampus. 2021 Jul;31(7):701-716. doi: 10.1002/hipo.23316. Epub 2021 Feb 19. Hippocampus. 2021. PMID: 33606338 Free PMC article.
-
Rodent age-related impairments in discriminating perceptually similar objects parallel those observed in humans.Hippocampus. 2017 Jul;27(7):759-776. doi: 10.1002/hipo.22729. Epub 2017 Apr 18. Hippocampus. 2017. PMID: 28342259 Free PMC article.
-
Cardiorespiratory fitness, hippocampal subfield volumes, and mnemonic discrimination task performance in aging.Hum Brain Mapp. 2021 Mar;42(4):871-892. doi: 10.1002/hbm.25259. Epub 2020 Dec 16. Hum Brain Mapp. 2021. PMID: 33325614 Free PMC article. Clinical Trial.
-
Mnemonic Similarity Task: A Tool for Assessing Hippocampal Integrity.Trends Cogn Sci. 2019 Nov;23(11):938-951. doi: 10.1016/j.tics.2019.08.003. Epub 2019 Oct 6. Trends Cogn Sci. 2019. PMID: 31597601 Free PMC article. Review.
-
Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics.Neurobiol Learn Mem. 2016 Mar;129:38-49. doi: 10.1016/j.nlm.2015.10.008. Epub 2015 Oct 26. Neurobiol Learn Mem. 2016. PMID: 26514299 Free PMC article. Review.
Cited by
-
The effects of moderate prenatal alcohol exposure on performance in object and spatial discrimination tasks by adult male rats.Behav Brain Res. 2025 Feb 26;478:115324. doi: 10.1016/j.bbr.2024.115324. Epub 2024 Nov 7. Behav Brain Res. 2025. PMID: 39521144
-
Perforant Path Fiber Loss Results in Mnemonic Discrimination Task Deficits in Young Rats.Front Syst Neurosci. 2018 Dec 11;12:61. doi: 10.3389/fnsys.2018.00061. eCollection 2018. Front Syst Neurosci. 2018. PMID: 30618655 Free PMC article.
-
Muscimol inactivation of dorsal striatum in young and aged male rats does not affect paired associates learning performance.Behav Neurosci. 2023 Dec;137(6):356-363. doi: 10.1037/bne0000561. Epub 2023 Jun 15. Behav Neurosci. 2023. PMID: 37326524 Free PMC article.
-
Unilateral Perforant Path Transection Does Not Alter Lateral Entorhinal Cortical or Hippocampal CA3 Arc Expression.Front Syst Neurosci. 2022 Jun 30;16:920713. doi: 10.3389/fnsys.2022.920713. eCollection 2022. Front Syst Neurosci. 2022. PMID: 35844245 Free PMC article.
-
Not the same as it ever was: A review of memory modification, updating, and distortion in humans and rodents.Neurosci Biobehav Rev. 2025 Jul;174:106195. doi: 10.1016/j.neubiorev.2025.106195. Epub 2025 May 3. Neurosci Biobehav Rev. 2025. PMID: 40324709 Review.
References
-
- Amaral D. G., Lavenex P. (2007). “Hippocampal neuroanatomy,” in The Hippocampus Book, eds Andersen P., Morris R. G., Amaral D. G., Bliss T. V., O’Keefe J. (Oxford: Oxford University Press; ), 37–131.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

