miR-26 Induces Apoptosis and Inhibits Autophagy in Non-small Cell Lung Cancer Cells by Suppressing TGF-β1-JNK Signaling Pathway
- PMID: 30687089
- PMCID: PMC6333751
- DOI: 10.3389/fphar.2018.01509
miR-26 Induces Apoptosis and Inhibits Autophagy in Non-small Cell Lung Cancer Cells by Suppressing TGF-β1-JNK Signaling Pathway
Abstract
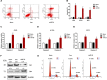
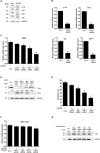
Non-small cell lung cancer (NSCLC) is one of the causes of cancer mortality worldwide. The role of miR-26 in the development and progression of NSCLC remains largely unknown. In this study we found an abnormal expression of miR-26 in human NSCLC tissues. It was found that miR-26 mimics induced cell apoptosis and promoted caspase-3, 9 activities in human NSCLC cells. The miR-26 inhibitor enhanced the expression of the light chain 3 (LC3) protein and the autophagy related genes in NSCLC cells. Moreover, miR-26 regulated apoptosis and autophagy by inhibiting TGF-β expression in a JNK dependent manner. In addition, miR-26 mimics induced cell apoptosis, was involved in the endoplasmic reticulum stress (ERS) signaling pathway. Down-regulation of the ERS, inhibited apoptosis which was induced by miR-26 mimics in NSCLC cells. In in vivo studies, TUNEL staining revealed that the number of TUNEL positive cells of the tumor tissue in the miR-26 treatment group, were significantly increased in comparison with the control group, while the number of TUNEL positive cells in the tumor tissue were remarkably decreased in the groups treated with miR-26, combined with the TGF-β1 inhibitor or JNK inhibitor. Additionally, the immunoreactivity of TGF-β1 in the cells treated with the miR-26 inhibitor, decreased in comparison to the control group. Our results indicated that miR-26 induced apoptosis and inhibited autophagy in human NSCLC cells through the TGF-β1-JNK signaling pathway, suggesting that miR-26 could be a potential novel target for the treatment of NSCLC.
Keywords: JNK; NSCLC; TGF-β; apoptosis; autophagy; miR-26.
Figures





Similar articles
-
miR-16 mimics inhibit TGF-β1-induced epithelial-to-mesenchymal transition via activation of autophagy in non-small cell lung carcinoma cells.Oncol Rep. 2018 Jan;39(1):247-254. doi: 10.3892/or.2017.6088. Epub 2017 Nov 9. Oncol Rep. 2018. PMID: 29138833
-
MiR-384 induces apoptosis and autophagy of non-small cell lung cancer cells through the negative regulation of Collagen α-1(X) chain gene.Biosci Rep. 2019 Feb 1;39(2):BSR20181523. doi: 10.1042/BSR20181523. Print 2019 Feb 28. Biosci Rep. 2019. PMID: 30442874 Free PMC article.
-
Effect of miR-21 on Apoptosis in Lung Cancer Cell Through Inhibiting the PI3K/ Akt/NF-κB Signaling Pathway in Vitro and in Vivo.Cell Physiol Biochem. 2018;46(3):999-1008. doi: 10.1159/000488831. Epub 2018 Apr 13. Cell Physiol Biochem. 2018. PMID: 29669316
-
MiR-9 is involved in TGF-β1-induced lung cancer cell invasion and adhesion by targeting SOX7.J Cell Mol Med. 2017 Sep;21(9):2000-2008. doi: 10.1111/jcmm.13120. Epub 2017 Mar 7. J Cell Mol Med. 2017. PMID: 28266181 Free PMC article.
-
Natural products targeting autophagy and apoptosis in NSCLC: a novel therapeutic strategy.Front Oncol. 2024 Apr 2;14:1379698. doi: 10.3389/fonc.2024.1379698. eCollection 2024. Front Oncol. 2024. PMID: 38628670 Free PMC article. Review.
Cited by
-
Free circulating mircoRNAs support the diagnosis of invasive aspergillosis in patients with hematologic malignancies and neutropenia.Sci Rep. 2020 Oct 5;10(1):16532. doi: 10.1038/s41598-020-73556-5. Sci Rep. 2020. PMID: 33020578 Free PMC article.
-
MicroRNA biomarkers as next-generation diagnostic tools for neurodegenerative diseases: a comprehensive review.Front Mol Neurosci. 2024 May 31;17:1386735. doi: 10.3389/fnmol.2024.1386735. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38883980 Free PMC article. Review.
-
The translational potential of miR-26 in atherosclerosis and development of agents for its target genes ACC1/2, COL1A1, CPT1A, FBP1, DGAT2, and SMAD7.Cardiovasc Diabetol. 2024 Jan 9;23(1):21. doi: 10.1186/s12933-024-02119-z. Cardiovasc Diabetol. 2024. PMID: 38195542 Free PMC article. Review.
-
Role of MicroRNAs in Parkinson's Disease.Int J Mol Sci. 2019 Nov 12;20(22):5649. doi: 10.3390/ijms20225649. Int J Mol Sci. 2019. PMID: 31718095 Free PMC article. Review.
-
An Integrated Strategy for Effective-Component Discovery of Astragali Radix in the Treatment of Lung Cancer.Front Pharmacol. 2021 Jan 14;11:580978. doi: 10.3389/fphar.2020.580978. eCollection 2020. Front Pharmacol. 2021. PMID: 33628171 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

