Selenium-Containing Protein From Selenium-Enriched Spirulina platensis Attenuates Cisplatin-Induced Apoptosis in MC3T3-E1 Mouse Preosteoblast by Inhibiting Mitochondrial Dysfunction and ROS-Mediated Oxidative Damage
- PMID: 30687122
- PMCID: PMC6333850
- DOI: 10.3389/fphys.2018.01907
Selenium-Containing Protein From Selenium-Enriched Spirulina platensis Attenuates Cisplatin-Induced Apoptosis in MC3T3-E1 Mouse Preosteoblast by Inhibiting Mitochondrial Dysfunction and ROS-Mediated Oxidative Damage
Abstract
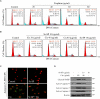
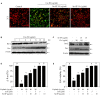
Accumulated evidences have verified that cancer chemotherapy may increase the risk of osteoporosis and severely affected the life quality. Osteoclasts hyperactivation was commonly accepted as the major pathogenesis of osteoporosis. However, the role of osteoblasts dysfunction in osteoporosis was little investigated. Our previous study has confirmed that selenium-containing protein from selenium-enriched Spirulina platensis (Se-SP) exhibited enhanced hepatoprotective potential through inhibiting oxidative damage. Herein, the protective effect of Se-SP against cisplatin-induced osteoblasts dysfunction in MC3T3-E1 mouse preosteoblast was investigated, and the underlying mechanism was evaluated. The results indicated that cisplatin dramatically decreased cell viability of preosteoblast by triggering mitochondria-mediated apoptosis pathway. Cisplatin treatment also caused mitochondrial dysfunction and reactive oxide species (ROS)-mediated oxidative damage. However, Se-SP pre-treatment effectively prevented MC3T3-E1 cells from cisplatin-induced mitochondrial dysfunction by balancing Bcl-2 family expression and regulating the opening of mitochondrial permeability transition pore (MPTP), attenuated cisplatin-induced oxidative damage through inhibiting the overproduction of ROS and superoxide anion, and eventually reversed cisplating-induced early and late apoptosis by inhibiting PARP cleavage and caspases activation. Our findings validated that Se-SP as a promising Se species could be a highly effective way in the chemoprevention and chemotherapy of oxidative damage-mediated bone diseases.
Keywords: apoptosis; cancer chemotherapy; mitochondrial dysfunction; osteoblasts dysfunction; osteoporosis; oxidative damage; selenium-containing protein.
Figures



References
LinkOut - more resources
Full Text Sources

