Self-Reported Fatigue After Mild Traumatic Brain Injury Is Not Associated With Performance Fatigability During a Sustained Maximal Contraction
- PMID: 30687127
- PMCID: PMC6335345
- DOI: 10.3389/fphys.2018.01919
Self-Reported Fatigue After Mild Traumatic Brain Injury Is Not Associated With Performance Fatigability During a Sustained Maximal Contraction
Abstract
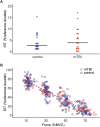
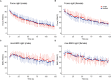
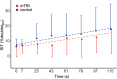
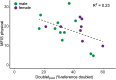
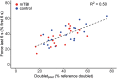
Patients with mild traumatic brain injury (mTBI) are frequently affected by fatigue. However, hardly any data is available on the fatigability of the motor system. We evaluated fatigue using the Fatigue Severity Scale (FSS) and Modified Fatigue Impact Scale (MFIS) questionnaires in 20 participants with mTBI (>3 months post injury; 8 females) and 20 age- and sex matched controls. Furthermore, index finger abduction force and electromyography of the first dorsal interosseous muscle of the right hand were measured during brief and sustained maximal voluntary contractions (MVC). Double pulse stimulation (100 Hz) was applied to the ulnar nerve to evoke doublet-forces before and after the sustained contraction. Seven superimposed twitches were evoked during the sustained MVC to quantify voluntary muscle activation. mTBI participants reported higher FSS scores (mTBI: 5.2 ± 0.8 SD vs. control: 2.8 ± 0.8 SD; P < 0.01). During the sustained MVC, force declined to similar levels in mTBI (30.0 ± 9.9% MVC) and control participants (32.7 ± 9.8% MVC, P = 0.37). The decline in doublet-forces after the sustained MVC (mTBI: to 37.2 ± 12.1 vs. control: to 41.4 ± 14.0% reference doublet, P = 0.32) and the superimposed twitches evoked during the sustained MVC (mTBI: median 9.3, range: 2.2-32.9 vs. control: median 10.3, range: 1.9-31.0% doubletpre, P = 0.34) also did not differ between groups. Force decline was associated with decline in doublet-force (R 2 = 0.50, P < 0.01) for both groups. Including a measure of voluntary muscle activation resulted in more explained variance for mTBI participants only. No associations between self-reported fatigue and force decline or voluntary muscle activation were found in mTBI participants. However, the physical subdomain of the MFIS was associated with the decline in doublet-force after the sustained MVC (R 2 = 0.23, P = 0.04). These results indicate that after mTBI, increased levels of self-reported physical fatigue reflected increased fatigability due to changes in peripheral muscle properties, but not force decline or muscle activation. Additionally, muscle activation was more important to explain the decline in voluntary force (performance fatigability) after mTBI than in control participants.
Keywords: first dorsal interosseous; force decline; interpolated twitch technique; mTBI; motor task; voluntary muscle activation.
Figures

References
LinkOut - more resources
Full Text Sources

