Preparation of Clathrin-Coated Vesicles From Arabidopsis thaliana Seedlings
- PMID: 30687367
- PMCID: PMC6334190
- DOI: 10.3389/fpls.2018.01972
Preparation of Clathrin-Coated Vesicles From Arabidopsis thaliana Seedlings
Abstract
Clathrin coated vesicles (CCVs) mediate endocytosis of plasma membrane proteins and deliver their content to the endosomes for either subsequent recycling to the plasma membrane or transport to the vacuole for degradation. CCVs assemble also at the trans-Golgi network (TGN) and is responsible for the transport of proteins to other membranes. Oligomerization of clathrin and recruitment of adaptor protein complexes promote the budding and the release of CCVs. However, many of the details during plant CCV formation are not completely elucidated. The analysis of isolated CCVs is therefore important to better understand the formation of plant CCVs, their cargos and the regulation of clathrin-mediated transport processes. In this article, we describe an optimized method to isolate CCVs from Arabidopsis thaliana seedlings.
Keywords: Arabidopsis thaliana; clathrin coated vesicles; density fractionation; negative staining; scanning electron microscopy.
Figures
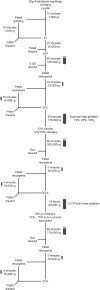

References
-
- Depta H., Robinson D. G. (1986). The isolation and enrichment of coated vesicles from suspension-cultured carrot cells. Protoplasma 130 162–170. 10.1007/BF01276598 - DOI
-
- Harley S. M., Beevers L. (1989). Isolation and partial characterization of clathrin-coated vesicles from pea (Pisum sativum L.) cotyledons. Protoplasma 150 103–109. 10.1007/BF01403665 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

