OmpA-Like Proteins of Porphyromonas gingivalis Mediate Resistance to the Antimicrobial Peptide LL-37
- PMID: 30687554
- PMCID: PMC6327258
- DOI: 10.1155/2018/2068435
OmpA-Like Proteins of Porphyromonas gingivalis Mediate Resistance to the Antimicrobial Peptide LL-37
Abstract
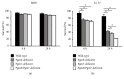
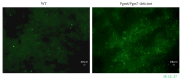
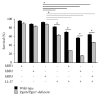
Subgingival bacteria are continually exposed to gingival crevicular fluids that are derived from serum, which contain various bactericidal agents. The periodontopathic bacterium Porphyromonas gingivalis has been demonstrated to possess a variety of abilities to resist bactericidal agents, due to which it is able to propagate in the subgingival environment. We previously demonstrated that the major surface glycoproteins of P. gingivalis-Pgm6 and Pgm7, also called outer membrane protein A-like proteins (OmpALPs)-mediate resistance to the bactericidal activity of human serum, but their precise role remains unknown. In this study, we investigated the sensitivity of the wild-type and Pgm6/Pgm7-deficient P. gingivalis strains toward major antimicrobial peptides in the oral cavity, human β-defensins (hBDs) 1-3, and human cathelicidin LL-37. hBDs showed a considerably weak bactericidal activity against both bacterial strains. LL-37 also showed a weak activity against the wild-type strain; however, it showed a significant activity against the Pgm6/Pgm7-deficient strain. In the Pgm6/Pgm7-deficient strain, LL-37 remarkably accumulated on the bacterial cell surface, which may result in the destruction of the outer membrane. Additionally, the bactericidal activity of hBDs against the Pgm6/Pgm7-deficient strain was found to be synergistically promoted in the presence of LL-37. Our results suggest that OmpALPs specifically protect P. gingivalis from the bactericidal activity of LL-37; thus, P. gingivalis may adeptly survive in LL-37-producing subgingival environments.
Figures

Similar articles
-
OmpA-like proteins of Porphyromonas gingivalis contribute to serum resistance and prevent Toll-like receptor 4-mediated host cell activation.PLoS One. 2018 Aug 28;13(8):e0202791. doi: 10.1371/journal.pone.0202791. eCollection 2018. PLoS One. 2018. PMID: 30153274 Free PMC article.
-
Trimeric structure of major outer membrane proteins homologous to OmpA in Porphyromonas gingivalis.J Bacteriol. 2005 Feb;187(3):902-11. doi: 10.1128/JB.187.3.902-911.2005. J Bacteriol. 2005. PMID: 15659668 Free PMC article.
-
Characterization of wheat germ agglutinin lectin-reactive glycosylated OmpA-like proteins derived from Porphyromonas gingivalis.Infect Immun. 2014 Nov;82(11):4563-71. doi: 10.1128/IAI.02069-14. Epub 2014 Aug 18. Infect Immun. 2014. PMID: 25135681 Free PMC article.
-
Porphyromonas gingivalis gingipains: the molecular teeth of a microbial vampire.Curr Protein Pept Sci. 2003 Dec;4(6):409-26. doi: 10.2174/1389203033487009. Curr Protein Pept Sci. 2003. PMID: 14683427 Review.
-
Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors.Crit Rev Oral Biol Med. 2002;13(2):132-42. doi: 10.1177/154411130201300204. Crit Rev Oral Biol Med. 2002. PMID: 12097356 Review.
Cited by
-
Interactions Between Neutrophils and Periodontal Pathogens in Late-Onset Periodontitis.Front Cell Infect Microbiol. 2021 Mar 12;11:627328. doi: 10.3389/fcimb.2021.627328. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33777839 Free PMC article. Review.
-
Illuminating the oral microbiome: cellular microbiology.FEMS Microbiol Rev. 2023 Jul 5;47(4):fuad045. doi: 10.1093/femsre/fuad045. FEMS Microbiol Rev. 2023. PMID: 37533213 Free PMC article.
-
Antimicrobial Peptide Synergies for Fighting Infectious Diseases.Adv Sci (Weinh). 2023 Sep;10(26):e2300472. doi: 10.1002/advs.202300472. Epub 2023 Jul 5. Adv Sci (Weinh). 2023. PMID: 37407512 Free PMC article. Review.
-
Outer Membrane Porins Contribute to Antimicrobial Resistance in Gram-Negative Bacteria.Microorganisms. 2023 Jun 28;11(7):1690. doi: 10.3390/microorganisms11071690. Microorganisms. 2023. PMID: 37512863 Free PMC article. Review.
-
Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review.Microorganisms. 2024 Jun 21;12(7):1259. doi: 10.3390/microorganisms12071259. Microorganisms. 2024. PMID: 39065030 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

