ALK Inhibitors in the Treatment of ALK Positive NSCLC
- PMID: 30687633
- PMCID: PMC6333640
- DOI: 10.3389/fonc.2018.00557
ALK Inhibitors in the Treatment of ALK Positive NSCLC
Abstract
Background: ALK inhibitors have shown positive advance in the treatment of ALK+ NSCLC. They have achieved better results in prolonging the progression free survival and improving quality of life in comparison to chemotherapy. We have assembled the evidence related to the efficacy and safety of these agents in the treatment of ALK positive NSCLC. Materials and Methods: A comprehensive search was conducted using electronic databases of PubMed, Medline and Cochrane Library to identify the studies involving comparison of ALK inhibitors to chemotherapy and Next generation ALK inhibitors to crizotinib. PFS was the primary outcome while other outcomes like ORR, adverse events, quality of life and OS were also analyzed and compared. Hazard ratios and odds ratios obtained were analyzed using fixed effect or random effects model in Review Manager Software. Results: A total of 12 studies (n = 3,297) met the criteria for inclusion in this review and meta-analysis. ALK inhibitors including crizotinib, ceritinib and alectinib revealed significantly better PFS (HR 0.42 [0.35, 0.50; p < 0.00001]), ORR (Overall OR 6.59 [4.86, 8.94; p < 0.00001] as compared to chemotherapy in the first line as well as second line treatment settings. Intracranial response rate was better with ALK inhibitors (ceritinib and alectinib) as compared to chemotherapy OR 6.51 [2.86, 14.83; p < 0.00001]. No significant increase in grade 3 or 4 adverse events was observed with crizotinib (OR 1.21 [0.82, 1.77; p = 0.34]) or ceritinib (OR 1.49 [0.86, 2.57; p = 0.17]) when compared to chemotherapy individually. Quality of life indicators assessed were significantly improved with ALK inhibitors. Next generation agents (ceritinib, alectinib and brigatinib) revealed significant improvement in PFS (HR 0.50 [0.43, 0.57; p < 0.00001]), ORR (OR 1.57 [1.21, 2.04; p = 0.0006]) in comparison to crizotinib. Next generation agents (Alectinib and brigatinib) yielded better response intra-cranially than crizotinib in terms of objective response rate (OR 5.87 [3.49, 9.87; p < 0.00001]) and time to CNS progression (HR 0.25 [0.13, 0.46; p < 0.0001]). Alectinib by far resulted in fewer adverse events than chemotherapy or crizotinib. Conclusions: Overall ALK inhibitors are safe and effective treatment option in ALK+ non-small cell lung cancer. Of the ALK inhibitors, Next generation agents in particular alectinib and brigatinib are safer and more effective intra-cranially and can be preferred as first option.
Keywords: anaplastic lymphoma kinase (ALK); chemotherapy; molecular targeted agents; non-small cell lung cancer (NSCLC); progression free survival (PFS); quality of life (Qol).
Figures


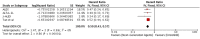

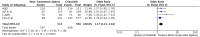






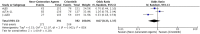

References
-
- Giovannetti EA., Rodriguez J. Targeted therapies in cancer: where are we going? Cancer Drug Resist. (2018) 1:82–6. 10.20517/cdr.2018.05 - DOI
-
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. (2011) 6:244–85. 10.1097/JTO.0b013e318206a221 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

