Transactivated Epidermal Growth Factor Receptor Recruitment of α-actinin-4 From F-actin Contributes to Invasion of Brain Microvascular Endothelial Cells by Meningitic Escherichia coli
- PMID: 30687645
- PMCID: PMC6333852
- DOI: 10.3389/fcimb.2018.00448
Transactivated Epidermal Growth Factor Receptor Recruitment of α-actinin-4 From F-actin Contributes to Invasion of Brain Microvascular Endothelial Cells by Meningitic Escherichia coli
Abstract
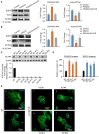
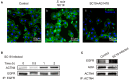
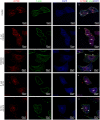
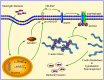
Bacterial penetration of the blood-brain barrier requires its successful invasion of brain microvascular endothelial cells (BMECs), and host actin cytoskeleton rearrangement in these cells is a key prerequisite for this process. We have reported previously that meningitic Escherichia coli can induce the activation of host's epidermal growth factor receptor (EGFR) to facilitate its invasion of BMECs. However, it is unknown how EGFR specifically functions during this invasion process. Here, we identified an important EGFR-interacting protein, α-actinin-4 (ACTN4), which is involved in maintaining and regulating the actin cytoskeleton. We observed that transactivated-EGFR competitively recruited ACTN4 from intracellular F-actin fibers to disrupt the cytoskeleton, thus facilitating bacterial invasion of BMECs. Strikingly, this mechanism operated not only for meningitic E. coli, but also for infections with Streptococcus suis, a Gram-positive meningitis-causing bacterial pathogen, thus revealing a common mechanism hijacked by these meningitic pathogens where EGFR competitively recruits ACTN4. Ever rising levels of antibiotic-resistant bacteria and the emergence of their extended-spectrum antimicrobial-resistant counterparts remind us that EGFR could act as an alternative non-antibiotic target to better prevent and control bacterial meningitis.
Keywords: bacterial meningitis; cytoskeleton; epidermal growth factor receptor; invasion; α-actinin-4.
Figures
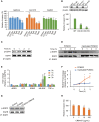
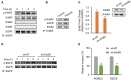
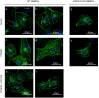
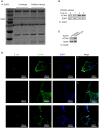
Similar articles
-
New insights into meningitic Escherichia coli infection of brain microvascular endothelial cells from quantitative proteomics analysis.J Neuroinflammation. 2018 Oct 19;15(1):291. doi: 10.1186/s12974-018-1325-z. J Neuroinflammation. 2018. PMID: 30340642 Free PMC article.
-
Sphingosine 1-Phosphate Activation of EGFR As a Novel Target for Meningitic Escherichia coli Penetration of the Blood-Brain Barrier.PLoS Pathog. 2016 Oct 6;12(10):e1005926. doi: 10.1371/journal.ppat.1005926. eCollection 2016 Oct. PLoS Pathog. 2016. PMID: 27711202 Free PMC article.
-
EGFR transactivation contributes to neuroinflammation in Streptococcus suis meningitis.J Neuroinflammation. 2016 Oct 19;13(1):274. doi: 10.1186/s12974-016-0734-0. J Neuroinflammation. 2016. PMID: 27756321 Free PMC article.
-
Invasion processes of pathogenic Escherichia coli.Int J Med Microbiol. 2005 Oct;295(6-7):463-70. doi: 10.1016/j.ijmm.2005.07.004. Int J Med Microbiol. 2005. PMID: 16238020 Review.
-
Strategy of Escherichia coli for crossing the blood-brain barrier.J Infect Dis. 2002 Dec 1;186 Suppl 2:S220-4. doi: 10.1086/344284. J Infect Dis. 2002. PMID: 12424701 Review.
Cited by
-
Open source board based acoustofluidic transwells for reversible disruption of the blood-brain barrier for therapeutic delivery.Biomater Res. 2023 Jul 15;27(1):69. doi: 10.1186/s40824-023-00406-6. Biomater Res. 2023. PMID: 37452381 Free PMC article.
-
Specific Integration of Temperate Phage Decreases the Pathogenicity of Host Bacteria.Front Cell Infect Microbiol. 2020 Feb 4;10:14. doi: 10.3389/fcimb.2020.00014. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32117795 Free PMC article.
-
LncRNA TARID induces cell proliferation through cell cycle pathway associated with coronary artery disease.Mol Biol Rep. 2022 Jun;49(6):4573-4581. doi: 10.1007/s11033-022-07304-5. Epub 2022 Mar 18. Mol Biol Rep. 2022. PMID: 35304681
-
PHF23 promotes NSCLC proliferation, metastasis, and chemoresistance via stabilization of ACTN4 and activation of the ERK pathway.Cell Death Dis. 2023 Aug 25;14(8):558. doi: 10.1038/s41419-023-06069-4. Cell Death Dis. 2023. PMID: 37626047 Free PMC article.
-
Effect of very low-protein diets supplemented with branched-chain amino acids on energy balance, plasma metabolomics and fecal microbiome of pigs.Sci Rep. 2020 Sep 28;10(1):15859. doi: 10.1038/s41598-020-72816-8. Sci Rep. 2020. PMID: 32985541 Free PMC article.
References
-
- Adams R., Brown E., Brown L., Butler R., Falk S., Fisher D., et al. . (2017). Inhibition of EGFR, HER2, and HER3 signalling in patients with colorectal cancer wild-type for BRAF, PIK3CA, KRAS, and NRAS (FOCUS4-D): a phase 2-3 randomised trial. Lancet Gastroenterol. Hepatol. 3, 162–171. 10.1016/S2468-1253(17)30394-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

