Comparing the Role of Mechanical Forces in Vascular and Valvular Calcification Progression
- PMID: 30687719
- PMCID: PMC6335252
- DOI: 10.3389/fcvm.2018.00197
Comparing the Role of Mechanical Forces in Vascular and Valvular Calcification Progression
Abstract
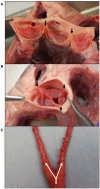
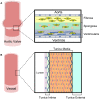
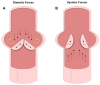
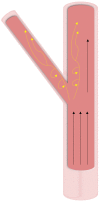
Calcification is a prevalent disease in most fully developed countries and is predominantly observed in heart valves and nearby vasculature. Calcification of either tissue leads to deterioration and, ultimately, failure causing poor quality of life and decreased overall life expectancy in patients. In valves, calcification presents as Calcific Aortic Valve Disease (CAVD), in which the aortic valve becomes stenotic when calcific nodules form within the leaflets. The initiation and progression of these calcific nodules is strongly influenced by the varied mechanical forces on the valve. In turn, the addition of calcific nodules creates localized disturbances in the tissue biomechanics, which affects extracellular matrix (ECM) production and cellular activation. In vasculature, atherosclerosis is the most common occurrence of calcification. Atherosclerosis exhibits as calcific plaque formation that forms in juxtaposition to areas of low blood shear stresses. Research in these two manifestations of calcification remain separated, although many similarities persist. Both diseases show that the endothelial layer and its regulation of nitric oxide is crucial to calcification progression. Further, there are similarities between vascular smooth muscle cells and valvular interstitial cells in terms of their roles in ECM overproduction. This review summarizes valvular and vascular tissue in terms of their basic anatomy, their cellular and ECM components and mechanical forces. Calcification is then examined in both tissues in terms of disease prediction, progression, and treatment. Highlighting the similarities and differences between these areas will help target further research toward disease treatment.
Keywords: CAVD; atherosclerosis; biomechanics; oscillatory stress; shear stress; valvular calcification; vascular calcification.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

