Transarterial Radioembolization (TARE) Agents beyond 90Y-Microspheres
- PMID: 30687734
- PMCID: PMC6330886
- DOI: 10.1155/2018/1435302
Transarterial Radioembolization (TARE) Agents beyond 90Y-Microspheres
Abstract
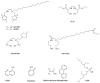
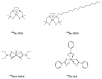
Liver malignancies, either primary tumours (mainly hepatocellular carcinoma and cholangiocarcinoma) or secondary hepatic metastases, are a major cause of death, with an increasing incidence. Among them, hepatocellular carcinoma (HCC) presents with a dark prognosis because of underlying liver diseases and an often late diagnosis. A curative surgical treatment can therefore only be proposed in 20 to 30% of the patients. However, new treatment options for intermediate to advanced stages, such as internal radionuclide therapy, seem particularly attractive. Transarterial radioembolization (TARE), which consists in the use of intra-arterial injection of a radiolabelled embolising agent, has led to very promising results. TARE with 90Y-loaded microspheres is now becoming an established procedure to treat liver tumours, with two commercially available products (namely, SIR-Sphere® and TheraSphere®). However, this technology remains expensive and is thus not available everywhere. The aim of this review is to describe TARE alternative technologies currently developed and investigated in clinical trials, with special emphasis on HCC.
Figures


References
-
- London W. T., McGlynn K. A. Liver cancer. In: Schottenfeld D., Fraumeni J., editors. Cancer Epidemiology and Prevention. 3rd. New York, NY, USA: Oxford University Press; 2006. pp. 763–786.
-
- Ahmed I., Lobo D. N. Malignant tumours of the liver. Surgery (Oxford) 2009;27(1):30–37. doi: 10.1016/j.mpsur.2008.12.005. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

