Recent Advances in HBV Reactivation Research
- PMID: 30687740
- PMCID: PMC6327272
- DOI: 10.1155/2018/2931402
Recent Advances in HBV Reactivation Research
Abstract
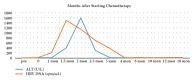
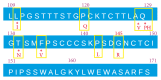
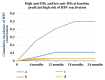
Hepatitis B virus (HBV) is an important public health problem that poses a serious threat to human health. HBV reactivation generally occurs in overt or occult HBV infection patients who suffered DDAs, chemotherapy, or immunosuppressive therapy, especially when some solid tumors and leukemia patients are using hormones such as prednisolone and imatinib. The approximate incidence of HBV reactivation ranged from about 10% to 40%. Scientists often explore the molecular mechanisms from both the virus and the host. But some studies have reported that some drugs (cisplatin, rituximab, imatinib, and glucocorticoid) could induce HBV reactivation directly. However, the specific molecular mechanisms were unclear. With the emergence of new antiviral drugs and molecular targeted drugs, the risk of HBV reactivation will increase significantly. Therefore this review was expected to be used to provide recommendations for future research in HBV reactivation.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

