Myricetin Protects Against High Glucose-Induced β-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5
- PMID: 30688049
- PMCID: PMC6470101
- DOI: 10.4093/dmj.2018.0052
Myricetin Protects Against High Glucose-Induced β-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5
Abstract
Background: Chronic hyperglycemia has deleterious effects on pancreatic β-cell function and turnover. Recent studies support the view that cyclin-dependent kinase 5 (CDK5) plays a role in β-cell failure under hyperglycemic conditions. However, little is known about how CDK5 impair β-cell function. Myricetin, a natural flavonoid, has therapeutic potential for the treatment of type 2 diabetes mellitus. In this study, we examined the effect of myricetin on high glucose (HG)-induced β-cell apoptosis and explored the relationship between myricetin and CDK5.
Methods: To address this question, we subjected INS-1 cells and isolated rat islets to HG conditions (30 mM) in the presence or absence of myricetin. Docking studies were conducted to validate the interaction between myricetin and CDK5. Gene expression and protein levels of endoplasmic reticulum (ER) stress markers were measured by real-time reverse transcription polymerase chain reaction and Western blot analysis.
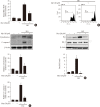
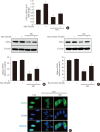
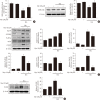
Results: Activation of CDK5 in response to HG coupled with the induction of ER stress via the down regulation of sarcoendoplasmic reticulum calcium ATPase 2b (SERCA2b) gene expression and reduced the nuclear accumulation of pancreatic duodenal homeobox 1 (PDX1) leads to β-cell apoptosis. Docking study predicts that myricetin inhibit CDK5 activation by direct binding in the ATP-binding pocket. Myricetin counteracted the decrease in the levels of PDX1 and SERCA2b by HG. Moreover, myricetin attenuated HG-induced apoptosis in INS-1 cells and rat islets and reduce the mitochondrial dysfunction by decreasing reactive oxygen species production and mitochondrial membrane potential (ΔΨm) loss.
Conclusion: Myricetin protects the β-cells against HG-induced apoptosis by inhibiting ER stress, possibly through inactivation of CDK5 and consequent upregulation of PDX1 and SERCA2b.
Keywords: Apoptosis; Cyclin-dependent kinase 5; Endoplasmic reticulum stress; Insulin-secreting cells; Myricetin.
Copyright © 2019 Korean Diabetes Association.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


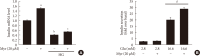
Similar articles
-
Myricetin prevents thapsigargin-induced CDK5-P66Shc signalosome mediated pancreatic β-cell dysfunction.Free Radic Biol Med. 2019 Sep;141:59-66. doi: 10.1016/j.freeradbiomed.2019.05.038. Epub 2019 Jun 1. Free Radic Biol Med. 2019. PMID: 31163256
-
Pancreatic and duodenal homeobox protein 1 (Pdx-1) maintains endoplasmic reticulum calcium levels through transcriptional regulation of sarco-endoplasmic reticulum calcium ATPase 2b (SERCA2b) in the islet β cell.J Biol Chem. 2014 Nov 21;289(47):32798-810. doi: 10.1074/jbc.M114.575191. Epub 2014 Sep 30. J Biol Chem. 2014. PMID: 25271154 Free PMC article.
-
PPAR-γ activation restores pancreatic islet SERCA2 levels and prevents β-cell dysfunction under conditions of hyperglycemic and cytokine stress.Mol Endocrinol. 2012 Feb;26(2):257-71. doi: 10.1210/me.2011-1181. Epub 2012 Jan 12. Mol Endocrinol. 2012. PMID: 22240811 Free PMC article.
-
Flavonoids, Isoflavonoids and others Bioactives for Insulin Sensitizations.Curr Diabetes Rev. 2024;20(2):e270423216247. doi: 10.2174/1573399819666230427095200. Curr Diabetes Rev. 2024. PMID: 37102490 Review.
-
Biophysical insights into glucose-dependent transcriptional regulation by PDX1.J Biol Chem. 2022 Dec;298(12):102623. doi: 10.1016/j.jbc.2022.102623. Epub 2022 Oct 20. J Biol Chem. 2022. PMID: 36272648 Free PMC article. Review.
Cited by
-
Natural Polyphenols as SERCA Activators: Role in the Endoplasmic Reticulum Stress-Related Diseases.Molecules. 2022 Aug 10;27(16):5095. doi: 10.3390/molecules27165095. Molecules. 2022. PMID: 36014327 Free PMC article. Review.
-
Myricetin Amplifies Glucose-Stimulated Insulin Secretion via the cAMP-PKA-Epac-2 Signaling Cascade.Biomedicines. 2025 Jun 12;13(6):1447. doi: 10.3390/biomedicines13061447. Biomedicines. 2025. PMID: 40564164 Free PMC article.
-
The pharmacological mechanism of Abelmoschus manihot in the treatment of chronic kidney disease.Heliyon. 2023 Nov 10;9(11):e22017. doi: 10.1016/j.heliyon.2023.e22017. eCollection 2023 Nov. Heliyon. 2023. PMID: 38058638 Free PMC article. Review.
-
Effects of myricetin against cadmium-induced neurotoxicity in PC12 cells.Toxicol Res (Camb). 2021 Jan 22;10(1):84-90. doi: 10.1093/toxres/tfaa104. eCollection 2021 Jan. Toxicol Res (Camb). 2021. PMID: 33613976 Free PMC article.
-
Research progress on the mechanism of beta-cell apoptosis in type 2 diabetes mellitus.Front Endocrinol (Lausanne). 2022 Aug 18;13:976465. doi: 10.3389/fendo.2022.976465. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36060972 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

