Early constraint-induced movement therapy affects behavior and neuronal plasticity in ischemia-injured rat brains
- PMID: 30688263
- PMCID: PMC6375040
- DOI: 10.4103/1673-5374.249225
Early constraint-induced movement therapy affects behavior and neuronal plasticity in ischemia-injured rat brains
Abstract
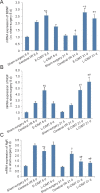
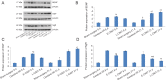
Constraint-induced movement therapy is an effective rehabilitative training technique used to improve the restoration of impaired upper extremity movement after stroke. However, whether constraint-induced movement therapy is more effective than conventional rehabilitation in acute or sub-acute stroke remains controversial. The aim of the present study was to identify the optimal time to start constraint-induced movement therapy after ischemic stroke and to explore the mechanisms by which constraint-induced movement therapy leads to post-stroke recovery. Sixty-four adult male Sprague-Dawley rats were randomly divided into four groups: sham-surgery group, cerebral ischemia/reperfusion group, early constraint-induced movement therapy group, and late constraint-induced movement therapy group. Rat models of left middle cerebral artery occlusion were established according to the Zea Longa line embolism method. Constraint-induced movement therapy was conducted starting on day 1 or day 14 in the early constraint-induced movement therapy and late constraint-induced movement therapy groups, respectively. To explore the effect of each intervention time on neuromotor function, behavioral function was assessed using a balance beam walking test before surgery and at 8 and 21 days after surgery. The expression levels of brain-derived neurotrophic factor, nerve growth factor and Nogo receptor were evaluated using real time-polymerase chain reaction and western blot assay to assess the effect of each intervention time. The results showed that the behavioral score was significantly lower in the early constraint-induced movement therapy group than in the cerebral ischemia/reperfusion and late constraint-induced movement therapy groups at 8 days. At 21 days, the scores had significantly decreased in the early constraint-induced movement therapy and late constraint-induced movement therapy groups. At 8 days, only mild pyknosis appeared in neurons of the ischemic penumbra in the early constraint-induced movement therapy group, which was distinctly better than in the cerebral ischemia/reperfusion group. At 21 days, only a few vacuolated cells were observed and no obvious inflammatory cells were visible in late constraint-induced movement therapy group, which was much better than at 8 days. The mRNA and protein expression levels of brain-derived neurotrophic factor and nerve growth factor were significantly higher, but expression levels of Nogo receptor were significantly lower in the early constraint-induced movement therapy group compared with the cerebral ischemia/reperfusion and late constraint-induced movement therapy groups at 8 days. The changes in expression levels at 21 days were larger but similar in both the early constraint-induced movement therapy and late constraint-induced movement therapy groups. Besides, the protein nerve growth factor level was higher in the late constraint-induced movement therapy group than in the early constraint-induced movement therapy group at 21 days. These results suggest that both early (1 day) and late (14 days) constraint-induced movement therapy induces molecular plasticity and facilitates functional recovery after ischemic stroke, as illustrated by the histology. The mechanism may be associated with downregulation of Nogo receptor expression and upregulation of brain-derived neurotrophic factor and nerve growth factor expression.
Keywords: constraint-induced movement therapy; functional recovery; ischemic stroke; nerve growth factors; nerve regeneration; neural regeneration; neuronal plasticity; rats; real time-polymerase chain reaction; rehabilitation; western blot assay.
Conflict of interest statement
None
Figures


References
-
- Azab M, Al-Jarrah M, Nazzal M, Maayah M, Sammour MA, Jamous M. Effectiveness of constraint-induced movement therapy (CIMT) as home-based therapy on Barthel Index in patients with chronic stroke. Top Stroke Rehabil. 2009;16:207–211. - PubMed
-
- Bachman J. Reverse-transcription PCR (RT-PCR) Methods Enzymol. 2013;530:67–74. - PubMed
-
- Berretta A, Tzeng YC, Clarkson AN. Post-stroke recovery: the role of activity-dependent release of brain-derived neurotrophic factor. Expert Rev Neurother. 2014;14:1335–1344. - PubMed
-
- Cheng S, Ma M, Ma Y, Wang Z, Xu G, Liu X. Combination therapy with intranasal NGF and electroacupuncture enhanced cell proliferation and survival in rats after stroke. Neurol Res. 2009;31:753–758. - PubMed
LinkOut - more resources
Full Text Sources

