Use of Electron Paramagnetic Resonance in Biological Samples at Ambient Temperature and 77 K
- PMID: 30688300
- PMCID: PMC8015406
- DOI: 10.3791/58461
Use of Electron Paramagnetic Resonance in Biological Samples at Ambient Temperature and 77 K
Abstract
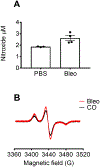
The accurate and specific detection of reactive oxygen species (ROS) in different cellular and tissue compartments is essential to the study of redox-regulated signaling in biological settings. Electron paramagnetic resonance spectroscopy (EPR) is the only direct method to assess free radicals unambiguously. Its advantage is that it detects physiologic levels of specific species with a high specificity, but it does require specialized technology, careful sample preparation, and appropriate controls to ensure accurate interpretation of the data. Cyclic hydroxylamine spin probes react selectively with superoxide or other radicals to generate a nitroxide signal that can be quantified by EPR spectroscopy. Cell-permeable spin probes and spin probes designed to accumulate rapidly in the mitochondria allow for the determination of superoxide concentration in different cellular compartments. In cultured cells, the use of cell permeable 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH) along with and without cell-impermeable superoxide dismutase (SOD) pretreatment, or use of cell-permeable PEG-SOD, allows for the differentiation of extracellular from cytosolic superoxide. The mitochondrial 1-hydroxy-4-[2-triphenylphosphonio)-acetamido]-2,2,6,6-tetramethyl-piperidine,1-hydroxy-2,2,6,6-tetramethyl-4-[2-(triphenylphosphonio)acetamido] piperidinium dichloride (mito-TEMPO-H) allows for measurement of mitochondrial ROS (predominantly superoxide). Spin probes and EPR spectroscopy can also be applied to in vivo models. Superoxide can be detected in extracellular fluids such as blood and alveolar fluid, as well as tissues such as lung tissue. Several methods are presented to process and store tissue for EPR measurements and deliver intravenous 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine (CPH) spin probe in vivo. While measurements can be performed at room temperature, samples obtained from in vitro and in vivo models can also be stored at -80 °C and analyzed by EPR at 77 K. The samples can be stored in specialized tubing stable at -80 °C and run at 77 K to enable a practical, efficient, and reproducible method that facilitates storing and transferring samples.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
