Efficacy of metformin on postprandial plasma triglyceride concentration by administration timing in patients with type 2 diabetes mellitus: A randomized cross-over pilot study
- PMID: 30688410
- PMCID: PMC6717824
- DOI: 10.1111/jdi.13016
Efficacy of metformin on postprandial plasma triglyceride concentration by administration timing in patients with type 2 diabetes mellitus: A randomized cross-over pilot study
Abstract
Aims/introduction: Preprandial metformin administration significantly reduces postprandial plasma triglyceride levels in animal studies by reducing intestinal absorption through delayed gastric emptying. However, this effect has not been shown in a clinical study. Therefore, we planned to investigate the efficacy of preprandial metformin administration on postprandial hypertriglyceridemia and the related gastrointestinal effects in patients with type 2 diabetes mellitus.
Materials and methods: A total of 11 patients taking single-dose metformin at 500-1,000 mg, with non-fasting plasma triglyceride levels of 150-1,000 mg/dL, were recruited at a single university hospital. The difference between preprandial and postprandial metformin administration on postprandial hypertriglyceridemia was examined by a meal test. The gastrointestinal effects of metformin, including stomach heaviness, heartburn and satiety, were also assessed using a visual analog scale.
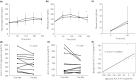
Results: The mean bodyweight of patients was 80.6 kg (body mass index 27.9 kg/m2 ), and the mean non-fasting plasma triglyceride level was 275.9 ± 57.0 mg/dL. The area under the curve of triglyceride during the meal test was significantly lower in the preprandial protocol than in the postprandial protocol (P < 0.05). Compared with postprandial administration, preprandial administration of metformin increased satiety (P = 0.036) without stomach heaviness or heartburn.
Conclusions: Preprandial metformin administration significantly reduced plasma triglyceride level during meal testing without marked exacerbation of gastrointestinal adverse effects. The present results suggest that a simple change in the timing of metformin administration represents a novel approach for enhancing triglyceride-lowering strategies in patients with type 2 diabetes mellitus and postprandial hypertriglyceridemia.
Keywords: Gastric emptying; Metformin; Postprandial hypertriglyceridemia.
© 2019 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures


Similar articles
-
Acute Effect of Metformin on Postprandial Hypertriglyceridemia through Delayed Gastric Emptying.Int J Mol Sci. 2017 Jun 16;18(6):1282. doi: 10.3390/ijms18061282. Int J Mol Sci. 2017. PMID: 28621714 Free PMC article.
-
Mealtime 50/50 basal + prandial insulin analogue mixture with a basal insulin analogue, both plus metformin, in the achievement of target HbA1c and pre- and postprandial blood glucose levels in patients with type 2 diabetes: a multinational, 24-week, randomized, open-label, parallel-group comparison.Clin Ther. 2007 Nov;29(11):2349-64. doi: 10.1016/j.clinthera.2007.11.016. Clin Ther. 2007. PMID: 18158076 Clinical Trial.
-
Impact of metformin versus the prandial insulin secretagogue, repaglinide, on fasting and postprandial glucose and lipid responses in non-obese patients with type 2 diabetes.Eur J Endocrinol. 2008 Jan;158(1):35-46. doi: 10.1530/EJE-07-0500. Eur J Endocrinol. 2008. PMID: 18166815 Clinical Trial.
-
Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review.
-
The effect of preprandial versus postprandial physical activity on glycaemia: Meta-analysis of human intervention studies.Diabetes Res Clin Pract. 2024 Apr;210:111638. doi: 10.1016/j.diabres.2024.111638. Epub 2024 Mar 27. Diabetes Res Clin Pract. 2024. PMID: 38548105 Review.
Cited by
-
Knowledge, Attitude, and Practice of Metformin Extended-Release Tablets Among Clinicians in China: A Cross-Sectional Survey.Front Pharmacol. 2021 Jul 12;12:634561. doi: 10.3389/fphar.2021.634561. eCollection 2021. Front Pharmacol. 2021. PMID: 34322016 Free PMC article.
-
X-box binding protein 1: A new metabolic mediator and drug target of metformin?Front Pharmacol. 2022 Nov 11;13:1013218. doi: 10.3389/fphar.2022.1013218. eCollection 2022. Front Pharmacol. 2022. PMID: 36438823 Free PMC article.
-
Role of the Gut in Diabetic Dyslipidemia.Front Endocrinol (Lausanne). 2020 Mar 13;11:116. doi: 10.3389/fendo.2020.00116. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32231641 Free PMC article. Review.
-
A new perspective on the biguanide, metformin therapy in type 2 diabetes and lactic acidosis.J Diabetes Investig. 2019 Jul;10(4):906-908. doi: 10.1111/jdi.13090. J Diabetes Investig. 2019. PMID: 31152685 Free PMC article.
References
-
- Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004; 291: 335–342. - PubMed
-
- Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014; 384: 625–635. - PubMed
-
- Simons LA, Dwyer T, Simons J, et al Chylomicrons and chylomicron remnants in coronary artery disease: a case‐control study. Atherosclerosis 1987; 65: 181–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

