Selective tissue targeting of synthetic nucleic acid drugs
- PMID: 30688661
- PMCID: PMC6391085
- DOI: 10.1172/JCI125228
Selective tissue targeting of synthetic nucleic acid drugs
Abstract
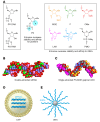
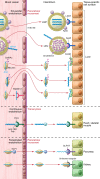
Antisense oligonucleotides (ASOs) are chemically synthesized nucleic acid analogs designed to bind to RNA by Watson-Crick base pairing. Following binding to the targeted RNA, the ASO perturbs RNA function by promoting selective degradation of the targeted RNA, altering RNA intermediary metabolism, or disrupting function of the RNA. Most antisense drugs are chemically modified to enhance their pharmacological properties and for passive targeting of the tissues of therapeutic interest. Recent advances in selective tissue targeting have resulted in a newer generation of ASO drugs that are more potent and better tolerated than previous generations, spawning renewed interest in identifying selective ligands that enhance targeted delivery of ASOs to tissues.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

