Prospective Validation of a Transcriptomic Metric in Severe Trauma
- PMID: 30688688
- PMCID: PMC6656642
- DOI: 10.1097/SLA.0000000000003204
Prospective Validation of a Transcriptomic Metric in Severe Trauma
Abstract
Objective:: To prospectively validate a previously discovered transcriptomic biomarker consisting of 63 blood leukocyte gene expression (S63) values to discriminate between trauma patients who rapidly recover and those with prolonged hospital stays who would benefit from early biological interventions.
Background:: Many severe trauma patients are successfully resuscitated but have complicated clinical trajectories leading to long-term functional, physical, and cognitive deficiencies. Identifying those trauma patients early would improve treatment plans and resource allocation. Unfortunately, current clinical scores and biomarkers used in trauma clinical trials have typically lacked adequate predictive ability.
Methods:: An independent, prospective, observational cohort study was performed involving 127 trauma subjects. The prospective cohort included patients admitted between October 2013 and August 2016 at 2 United States Level-1 trauma centers. An additional secondary analysis was performed using the Activation of Coagulation and Inflammation in Trauma (ACIT2) database of 26 trauma patients.
Results:: The S63 transcriptomic metric (AUC 0.80) outperformed clinical markers and plasma interleukin-6 for prospectively predicting trauma patients who require intensive care unit stays longer than 5 days with ongoing organ dysfunction. The same metric applied to an existing dataset (ACIT2) was similarly effective (AUC 0.85) at predicting multiorgan failure.
Conclusions:: A single transcriptomic metric of blood leukocyte gene expression can be used in blunt trauma cohorts at 24 hours to distinguish patients who rapidly recover from those with complicated clinical trajectories. The transcriptomic metric has been operationalized on an Food and Drug Administration 510(k)-cleared platform otherwise used for cancer diagnostics. This metric is only modestly improved when combined with clinical markers.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
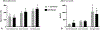


Comment in
-
Advancing Toward Precision Medicine in Trauma.Ann Surg. 2020 May;271(5):811-812. doi: 10.1097/SLA.0000000000003818. Ann Surg. 2020. PMID: 32301794 No abstract available.
References
-
- Iwashyna TJ, Hodgson CL, Pilcher D, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med. 2016;4:566–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM040586/GM/NIGMS NIH HHS/United States
- R01 GM104481/GM/NIGMS NIH HHS/United States
- T32 GM008721/GM/NIGMS NIH HHS/United States
- P30 AG028740/AG/NIA NIH HHS/United States
- P50 GM111152/GM/NIGMS NIH HHS/United States
- R01 GM081923/GM/NIGMS NIH HHS/United States
- R01 GM097531/GM/NIGMS NIH HHS/United States
- R01 GM040586/GM/NIGMS NIH HHS/United States
- R01 DK091443/DK/NIDDK NIH HHS/United States
- R01 GM113945/GM/NIGMS NIH HHS/United States
- P50 HL059412/HL/NHLBI NIH HHS/United States
- P01 HL059412/HL/NHLBI NIH HHS/United States
- R01 GM063041/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

