A dose ranging study of 2 different formulations of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants
- PMID: 30689507
- PMCID: PMC6988874
- DOI: 10.1080/21645515.2019.1568159
A dose ranging study of 2 different formulations of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants
Abstract
Background: Two new formulations of an investigational 15-valent pneumococcal conjugate vaccine (PCV15-A and PCV15-B) were developed using 2 different protein-polysaccharide conjugation processes and evaluated in separate phase I/II studies (NCT02037984 [V114-004] and NCT02531373 [V114-005]) to assess optimal concentrations of pneumococcal polysaccharide (PnPs) and Aluminum Phosphate Adjuvant.
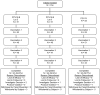
Methods: Various lots of PCV15-A and PCV15-B containing different concentrations of PnPs and/or adjuvant were compared to PCV13 in young adults and infants. Adults received single dose and infants received 4 doses at 2, 4, 6, and 12-15 months of age. Adverse events (AEs) were collected after each dose. Serotype-specific immunoglobulin G (IgG) concentrations and opsonophagocytic activity (OPA) were measured prior and 30 days postvaccination in adults, at 1 month postdose 3 (PD3), pre-dose4, and postdose 4 (PD4) in infants.
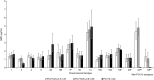
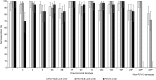
Results: Safety profiles were comparable across vaccination groups. At PD3, serotype-specific IgG GMCs were generally lower for either PCV15 formulation than PCV13 for most shared serotypes. PCV15 consistently elicited higher antibody responses to the 2 serotypes unique to the vaccine (22F and 33F) and serotype 3 for which PCV13 was shown to be ineffective. Except for serotypes 6A and 6B, no dose-response effect was observed with increasing concentrations of PnPs and/or adjuvant.
Conclusion: PCV15 is safe and induces IgG and OPA responses to all 15 serotypes in the vaccine. No significant differences in antibody responses were observed with increases in PnPs and/or Aluminum Phosphate Adjuvant.
Keywords: Pneumococcal conjugate vaccine; immunogenicity; safety.
Figures

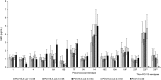

References
-
- Arditi M, Mason EO Jr, Bradley JS, Tan TQ, Barson WJ, Schutze GE, Wald ER, Givner LB, Kim KS, Yogev R, et al Three-year multicenter surveillance of pneumococcal meningitis in children: Clinical characteristics, and outcome related to penicillin susceptibility and dexamethasone use. Pediatrics. 1998;102(5):1087–1097. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
