Effectiveness of Seasonal Influenza Vaccination in Children in Senegal During a Year of Vaccine Mismatch: A Cluster-randomized Trial
- PMID: 30689757
- PMCID: PMC6821165
- DOI: 10.1093/cid/ciz066
Effectiveness of Seasonal Influenza Vaccination in Children in Senegal During a Year of Vaccine Mismatch: A Cluster-randomized Trial
Abstract
Background: The population effects of influenza vaccination in children have not been extensively studied, especially in tropical, developing countries. In rural Senegal, we assessed the total (primary objective) and indirect effectiveness of a trivalent inactivated influenza vaccine (IIV3).
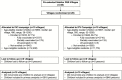
Methods: In this double-blind, cluster-randomized trial, villages were randomly allocated (1:1) for the high-coverage vaccination of children aged 6 months through 10 years with either the 2008-09 northern hemisphere IIV3 or an inactivated polio vaccine (IPV). Vaccinees were monitored for serious adverse events. All village residents, vaccinated and unvaccinated, were monitored for signs and symptoms of influenza illness using weekly home visits and surveillance in designated clinics. The primary outcome was all laboratory-confirmed symptomatic influenza.
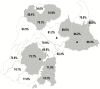
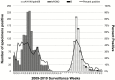
Results: Between 23 May and 11 July 2009, 20 villages were randomized, and 66.5% of age-eligible children were enrolled (3918 in IIV3 villages and 3848 in IPV villages). Follow-up continued until 28 May 2010. There were 4 unrelated serious adverse events identified. Among vaccinees, the total effectiveness against illness caused by the seasonal influenza virus (presumed to all be drifted A/H3N2, based on antigenic characterization data) circulating at high rates among children was 43.6% (95% confidence interval [CI] 18.6-60.9%). The indirect effectiveness against seasonal A/H3N2 was 15.4% (95% CI -22.0 to 41.3%). The total effectiveness against illness caused by the pandemic influenza virus (A/H1N1pdm09) was -52.1% (95% CI -177.2 to 16.6%).
Conclusions: IIV3 provided statistically significant, moderate protection to children in Senegal against circulating, pre-2010 seasonal influenza strains, but not against A/H1N1pdm09, which was not included in the vaccine. No indirect effects were measured. Further study in low-resource populations is warranted.
Clinical trials registration: NCT00893906.
Keywords: developing countries; herd immunity; human influenza; influenza vaccines; randomized controlled trial.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Evidence in a Cluster Randomized Controlled Trial of Increased 2009 Pandemic Risk Associated With 2008-2009 Seasonal Influenza Vaccine Receipt.Clin Infect Dis. 2019 Nov 27;69(12):2230-2231. doi: 10.1093/cid/ciz351. Clin Infect Dis. 2019. PMID: 31056676 Free PMC article. No abstract available.
-
Reply to Skowronski and De Serres.Clin Infect Dis. 2019 Nov 27;69(12):2231-2232. doi: 10.1093/cid/ciz352. Clin Infect Dis. 2019. PMID: 31056688 No abstract available.

