Rapid Molecular Tests for Influenza, Respiratory Syncytial Virus, and Other Respiratory Viruses: A Systematic Review of Diagnostic Accuracy and Clinical Impact Studies
- PMID: 30689772
- PMCID: PMC7108200
- DOI: 10.1093/cid/ciz056
Rapid Molecular Tests for Influenza, Respiratory Syncytial Virus, and Other Respiratory Viruses: A Systematic Review of Diagnostic Accuracy and Clinical Impact Studies
Abstract
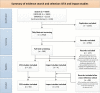
We systematically reviewed available evidence from Embase, Medline, and the Cochrane Library on diagnostic accuracy and clinical impact of commercially available rapid (results <3 hours) molecular diagnostics for respiratory viruses as compared to conventional molecular tests. Quality of included studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies criteria for diagnostic test accuracy (DTA) studies, and the Cochrane Risk of Bias Assessment and Risk of Bias in Nonrandomized Studies of Interventions criteria for randomized and observational impact studies, respectively. Sixty-three DTA reports (56 studies) were meta-analyzed with a pooled sensitivity of 90.9% (95% confidence interval [CI], 88.7%-93.1%) and specificity of 96.1% (95% CI, 94.2%-97.9%) for the detection of either influenza virus (n = 29), respiratory syncytial virus (RSV) (n = 1), influenza virus and RSV (n = 19), or a viral panel including influenza virus and RSV (n = 14). The 15 included impact studies (5 randomized) were very heterogeneous and results were therefore inconclusive. However, we suggest that implementation of rapid diagnostics in hospital care settings should be considered.
Keywords: diagnostic accuracy; impact; molecular diagnostics; rapid test; review.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Impact of Rapid Molecular Diagnostic Testing of Respiratory Viruses on Outcomes of Adults Hospitalized with Respiratory Illness: a Multicenter Quasi-experimental Study.J Clin Microbiol. 2019 Mar 28;57(4):e01727-18. doi: 10.1128/JCM.01727-18. Print 2019 Apr. J Clin Microbiol. 2019. PMID: 30541934 Free PMC article.
-
Comparison of the Xpert Flu/RSV XC and Xpress Flu/RSV Assays.J Clin Microbiol. 2018 Jul 26;56(8):e00278-18. doi: 10.1128/JCM.00278-18. Print 2018 Aug. J Clin Microbiol. 2018. PMID: 29769281 Free PMC article.
-
Evaluation of Seegene Allplex Respiratory Panel 1 kit for the detection of influenza virus and human respiratory syncytial virus.J Clin Virol. 2018 Aug;105:31-34. doi: 10.1016/j.jcv.2018.05.006. Epub 2018 May 25. J Clin Virol. 2018. PMID: 29883908 Free PMC article.
-
Summarizing Study Characteristics and Diagnostic Performance of Commercially Available Tests for Respiratory Syncytial Virus: A Scoping Literature Review in the COVID-19 Era.J Appl Lab Med. 2023 Mar 6;8(2):353-371. doi: 10.1093/jalm/jfac058. J Appl Lab Med. 2023. PMID: 35854475 Free PMC article.
-
Detection of Influenza A and B Viruses and Respiratory Syncytial Virus by Use of Clinical Laboratory Improvement Amendments of 1988 (CLIA)-Waived Point-of-Care Assays: a Paradigm Shift to Molecular Tests.J Clin Microbiol. 2018 Jun 25;56(7):e00367-18. doi: 10.1128/JCM.00367-18. Print 2018 Jul. J Clin Microbiol. 2018. PMID: 29695519 Free PMC article. Review.
Cited by
-
Advances and insights in the diagnosis of viral infections.J Nanobiotechnology. 2021 Oct 30;19(1):348. doi: 10.1186/s12951-021-01081-2. J Nanobiotechnology. 2021. PMID: 34717656 Free PMC article. Review.
-
Healthcare as a driver, reservoir and amplifier of antimicrobial resistance: opportunities for interventions.Nat Rev Microbiol. 2024 Oct;22(10):636-649. doi: 10.1038/s41579-024-01076-4. Epub 2024 Jul 24. Nat Rev Microbiol. 2024. PMID: 39048837 Review.
-
Evaluation of Diagnostic Tests.Methods Mol Biol. 2021;2249:319-333. doi: 10.1007/978-1-0716-1138-8_18. Methods Mol Biol. 2021. PMID: 33871852
-
Trends in Molecular Diagnosis of Nosocomial Pneumonia Classic PCR vs. Point-of-Care PCR: A Narrative Review.Healthcare (Basel). 2023 May 7;11(9):1345. doi: 10.3390/healthcare11091345. Healthcare (Basel). 2023. PMID: 37174887 Free PMC article. Review.
-
Implementation of point-of-care molecular testing for respiratory viruses in congregate living settings.Infect Control Hosp Epidemiol. 2024 Apr 25;45(9):1-5. doi: 10.1017/ice.2024.72. Online ahead of print. Infect Control Hosp Epidemiol. 2024. PMID: 38659123 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

