Central Mechanisms of Glucose Sensing and Counterregulation in Defense of Hypoglycemia
- PMID: 30689785
- PMCID: PMC6505456
- DOI: 10.1210/er.2018-00226
Central Mechanisms of Glucose Sensing and Counterregulation in Defense of Hypoglycemia
Abstract
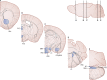
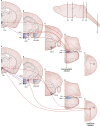
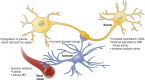
Glucose homeostasis requires an organism to rapidly respond to changes in plasma glucose concentrations. Iatrogenic hypoglycemia as a result of treatment with insulin or sulfonylureas is the most common cause of hypoglycemia in humans and is generally only seen in patients with diabetes who take these medications. The first response to a fall in glucose is the detection of impending hypoglycemia by hypoglycemia-detecting sensors, including glucose-sensing neurons in the hypothalamus and other regions. This detection is then linked to a series of neural and hormonal responses that serve to prevent the fall in blood glucose and restore euglycemia. In this review, we discuss the current state of knowledge about central glucose sensing and how detection of a fall in glucose leads to the stimulation of counterregulatory hormone and behavior responses. We also review how diabetes and recurrent hypoglycemia impact glucose sensing and counterregulation, leading to development of impaired awareness of hypoglycemia in diabetes.
Copyright © 2019 Endocrine Society.
Figures

References
-
- Khunti K, Alsifri S, Aronson R, Cigrovski Berković M, Enters-Weijnen C, Forsén T, Galstyan G, Geelhoed-Duijvestijn P, Goldfracht M, Gydesen H, Kapur R, Lalic N, Ludvik B, Moberg E, Pedersen-Bjergaard U, Ramachandran A; HAT Investigator Group. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18(9):907–915. - PMC - PubMed
-
- Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med. 2008;25(4):501–504. - PubMed
-
- Fukuda M, Ono T, Nishino H, Sasaki K. Independent glucose effects on rat hypothalamic neurons: an in vitro study. J Auton Nerv Syst. 1984;10(3–4):373–381. - PubMed
-
- Treherne JM, Ashford ML. Calcium-activated potassium channels in rat dissociated ventromedial hypothalamic neurons. J Neuroendocrinol. 1991;3(3):323–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

