The Arabidopsis AMOT1/EIN3 gene plays an important role in the amelioration of ammonium toxicity
- PMID: 30689938
- PMCID: PMC6382331
- DOI: 10.1093/jxb/ery457
The Arabidopsis AMOT1/EIN3 gene plays an important role in the amelioration of ammonium toxicity
Abstract
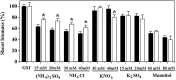
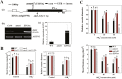
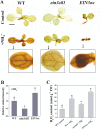
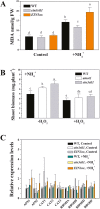
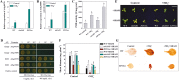
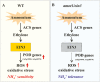
Ammonium (NH4+) toxicity inhibits shoot growth in Arabidopsis, but the underlying mechanisms remain poorly characterized. Here, we show that a novel Arabidopsis mutant, ammonium tolerance 1 (amot1), exhibits enhanced shoot growth tolerance to NH4+. Molecular cloning revealed that amot1 is a new allele of EIN3, a key regulator of ethylene responses. The amot1 mutant and the allelic ein3-1 mutants show greater NH4+ tolerance than the wild type. Moreover, transgenic plants overexpressing EIN3 (EIN3ox) are more sensitive to NH4+ toxicity The ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) increases shoot sensitivity to NH4+, whereas the ethylene perception inhibitor Ag+ decreases sensitivity. NH4+ induces ACC and ethylene accumulation. Furthermore, ethylene-insensitive mutants such as etr1-3 and ein3eil1 display enhanced NH4+ tolerance. In contrast, the ethylene overproduction mutant eto1-1 exhibits decreased ammonium tolerance. AMOT1/EIN3 positively regulates shoot ROS accumulation, leading to oxidative stress under NH4+ stress, a trait that may be related to increased expression of peroxidase-encoding genes. These findings demonstrate the role of AMOT1/EIN3 in NH4+ tolerance and confirm the strong link between NH4+ toxicity symptoms and the accumulation of hydrogen peroxide.
Keywords: amot1 mutant; AMOT1/EIN3; Ammonium stress; Arabidopsis; H2O2; peroxidases.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

