2-Aminothiazole Derivatives as Selective Allosteric Modulators of the Protein Kinase CK2. 2. Structure-Based Optimization and Investigation of Effects Specific to the Allosteric Mode of Action
- PMID: 30689946
- PMCID: PMC7579840
- DOI: 10.1021/acs.jmedchem.8b01765
2-Aminothiazole Derivatives as Selective Allosteric Modulators of the Protein Kinase CK2. 2. Structure-Based Optimization and Investigation of Effects Specific to the Allosteric Mode of Action
Abstract
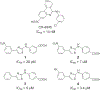
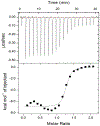
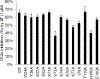
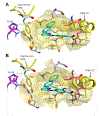
Protein CK2 has gained much interest as an anticancer drug target in the past decade. We had previously described the identification of a new allosteric site on the catalytic α-subunit, along with first small molecule ligands based on the 4-(4-phenylthiazol-2-ylamino)benzoic acid scaffold. In the present work, structure optimizations guided by a binding model led to the identification of the lead compound 2-hydroxy-4-((4-(naphthalen-2-yl)thiazol-2-yl)amino)benzoic acid (27), showing a submicromolar potency against purified CK2α (IC50 = 0.6 μM). Furthermore, 27 induced apoptosis and cell death in 786-O renal cell carcinoma cells (EC50 = 5 μM) and inhibited STAT3 activation even more potently than the ATP-competitive drug candidate CX-4945 (EC50 of 1.6 μM vs 5.3 μM). Notably, the potencies of our allosteric ligands to inhibit CK2 varied depending on the individual substrate. Altogether, the novel allosteric pocket was proved a druggable site, offering an excellent perspective to develop efficient and selective allosteric CK2 inhibitors.
Figures
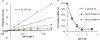




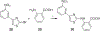
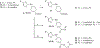
Similar articles
-
Identification and Biological Evaluation of CK2 Allosteric Fragments through Structure-Based Virtual Screening.Molecules. 2020 Jan 6;25(1):237. doi: 10.3390/molecules25010237. Molecules. 2020. PMID: 31935979 Free PMC article.
-
Recent Advances in the Discovery of CK2 Allosteric Inhibitors: From Traditional Screening to Structure-Based Design.Molecules. 2020 Feb 16;25(4):870. doi: 10.3390/molecules25040870. Molecules. 2020. PMID: 32079098 Free PMC article. Review.
-
2-Aminothiazole Derivatives as Selective Allosteric Modulators of the Protein Kinase CK2. 1. Identification of an Allosteric Binding Site.J Med Chem. 2019 Feb 28;62(4):1803-1816. doi: 10.1021/acs.jmedchem.8b01766. Epub 2019 Feb 18. J Med Chem. 2019. PMID: 30689953 Free PMC article.
-
Structural and Mechanistic Basis of the Inhibitory Potency of Selected 2-Aminothiazole Compounds on Protein Kinase CK2.J Med Chem. 2020 Jul 23;63(14):7766-7772. doi: 10.1021/acs.jmedchem.0c00587. Epub 2020 Jul 14. J Med Chem. 2020. PMID: 32589844
-
ATP site-directed inhibitors of protein kinase CK2: an update.Curr Top Med Chem. 2011;11(11):1340-51. doi: 10.2174/156802611795589638. Curr Top Med Chem. 2011. PMID: 21513497 Review.
Cited by
-
Development and therapeutic potential of 2-aminothiazole derivatives in anticancer drug discovery.Med Chem Res. 2021;30(4):771-806. doi: 10.1007/s00044-020-02686-2. Epub 2021 Jan 15. Med Chem Res. 2021. PMID: 33469255 Free PMC article. Review.
-
Identification and Biological Evaluation of CK2 Allosteric Fragments through Structure-Based Virtual Screening.Molecules. 2020 Jan 6;25(1):237. doi: 10.3390/molecules25010237. Molecules. 2020. PMID: 31935979 Free PMC article.
-
Downfalls of Chemical Probes Acting at the Kinase ATP-Site: CK2 as a Case Study.Molecules. 2021 Mar 31;26(7):1977. doi: 10.3390/molecules26071977. Molecules. 2021. PMID: 33807474 Free PMC article. Review.
-
Recent Advances in the Discovery of CK2 Allosteric Inhibitors: From Traditional Screening to Structure-Based Design.Molecules. 2020 Feb 16;25(4):870. doi: 10.3390/molecules25040870. Molecules. 2020. PMID: 32079098 Free PMC article. Review.
-
Proposed Allosteric Inhibitors Bind to the ATP Site of CK2α.J Med Chem. 2020 Nov 12;63(21):12786-12798. doi: 10.1021/acs.jmedchem.0c01173. Epub 2020 Oct 29. J Med Chem. 2020. PMID: 33119282 Free PMC article.
References
-
- Meggio F; Pinna LA, One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003, 17, 349–368. - PubMed
-
- Ruzzene M; Pinna LA, Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim. Biophys. Acta 2010, 1804, 499–504. - PubMed
-
- Siddiqui-Jain A; Drygin D; Streiner N; Chua P; Pierre F; O’Brien SE; Bliesath J; Omori M; Huser N; Ho C; Proffitt C; Schwaebe MK; Ryckman DM; Rice WG; Anderes K, CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010, 70, 10288–10298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

