Getting into Shape: Reflections on a New Generation of Cylindrical Nanostructures' Self-Assembly Using Polymer Building Blocks
- PMID: 30689954
- PMCID: PMC6407914
- DOI: 10.1021/jacs.8b08648
Getting into Shape: Reflections on a New Generation of Cylindrical Nanostructures' Self-Assembly Using Polymer Building Blocks
Abstract
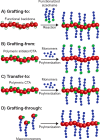
Cylinders are fascinating structures with uniquely high surface area, internal volume, and rigidity. On the nanoscale, a broad range of applications have demonstrated advantageous behavior of cylindrical micelles or bottlebrush polymers over traditional spherical nano-objects. In the past, obtaining pure samples of cylindrical nanostructures using polymer building blocks via conventional self-assembly strategies was challenging. However, in recent years, the development of advanced methods including polymerization-induced self-assembly, crystallization-driven self-assembly, and bottlebrush polymer synthesis has facilitated the easy synthesis of cylindrical nano-objects at industrially relevant scales. In this Perspective, we discuss these techniques in detail, highlighting the advantages and disadvantages of each strategy and considering how the cylindrical nanostructures that are obtained differ in their chemical structure, physical properties, colloidal stability, and reactivity. In addition, we propose future challenges to address in this rapidly expanding field.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

