Ocular adhesives: Design, chemistry, crosslinking mechanisms, and applications
- PMID: 30690421
- PMCID: PMC6687460
- DOI: 10.1016/j.biomaterials.2019.01.011
Ocular adhesives: Design, chemistry, crosslinking mechanisms, and applications
Abstract
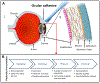
Closure of ocular wounds after an accident or surgery is typically performed by suturing, which is associated with numerous potential complications, including suture breakage, inflammation, secondary neovascularization, erosion to the surface and secondary infection, and astigmatism; for example, more than half of post-corneal transplant infections are due to suture related complications. Tissue adhesives provide promising substitutes for sutures in ophthalmic surgery. Ocular adhesives are not only intended to address the shortcomings of sutures, but also designed to be easy to use, and can potentially minimize post-operative complications. Herein, recent progress in the design, synthesis, and application of ocular adhesives, along with their advantages, limitations, and potential are discussed. This review covers two main classes of ocular adhesives: (1) synthetic adhesives based on cyanoacrylates, polyethylene glycol (PEG), and other synthetic polymers, and (2) adhesives based on naturally derived polymers, such as proteins and polysaccharides. In addition, different technologies to cover and protect ocular wounds such as contact bandage lenses, contact lenses coupled with novel technologies, and decellularized corneas are discussed. Continued advances in this area can help improve both patient satisfaction and clinical outcomes.
Keywords: Bioadhesives and sealants; Drug delivery; Natural and synthetic; Ocular.
Published by Elsevier Ltd.
Figures







References
-
- Acheson JF, Lyons CJ, Ocular Morbidity Due to Monofilament Nylon Corneal Sutures, Eye 5 (1991) 7. - PubMed
-
- Bhatia SS, Ocular surface sealants and adhesives, Ocul Surf 4(3) (2006) 146–54. - PubMed
-
- Chan SM, Boisjoly H.l.n., Advances in the use of adhesives in ophthalmology, Current Opinion in Ophthalmology 15(4) (2004) 305–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

