Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications
- PMID: 30691108
- PMCID: PMC6406734
- DOI: 10.3390/cells8020089
Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications
Abstract
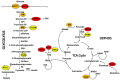
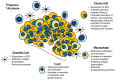
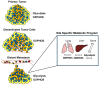
Current standard-of-care (SOC) therapy for breast cancer includes targeted therapies such as endocrine therapy for estrogen receptor-alpha (ERα) positive; anti-HER2 monoclonal antibodies for human epidermal growth factor receptor-2 (HER2)-enriched; and general chemotherapy for triple negative breast cancer (TNBC) subtypes. These therapies frequently fail due to acquired or inherent resistance. Altered metabolism has been recognized as one of the major mechanisms underlying therapeutic resistance. There are several cues that dictate metabolic reprogramming that also account for the tumors' metabolic plasticity. For metabolic therapy to be efficacious there is a need to understand the metabolic underpinnings of the different subtypes of breast cancer as well as the role the SOC treatments play in targeting the metabolic phenotype. Understanding the mechanism will allow us to identify potential therapeutic vulnerabilities. There are some very interesting questions being tackled by researchers today as they pertain to altered metabolism in breast cancer. What are the metabolic differences between the different subtypes of breast cancer? Do cancer cells have a metabolic pathway preference based on the site and stage of metastasis? How do the cell-intrinsic and -extrinsic cues dictate the metabolic phenotype? How do the nucleus and mitochondria coordinately regulate metabolism? How does sensitivity or resistance to SOC affect metabolic reprogramming and vice-versa? This review addresses these issues along with the latest updates in the field of breast cancer metabolism.
Keywords: breast cancer; estrogen receptors; metabolic reprogramming; metabolism; metabolism in metastatic cascade; mito-nuclear crosstalk; mitochondria; molecular subtypes; p53; precision medicine; resistance mechanisms; standard-of-care; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cairns R.A., Mak T.W. The current state of cancer metabolism. Nat. Rev. Cancer. 2016;16:613–614. doi: 10.1038/nrc.2016.100. - DOI
-
- LeBleu V.S., O’Connell J.T., Gonzalez Herrera K.N., Wikman H., Pantel K., Haigis M.C., de Carvalho F.M., Damascena A., Domingos Chinen L.T., Rocha R.M., et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014;16:992–1003. doi: 10.1038/ncb3039. - DOI - PMC - PubMed
-
- Dupuy F., Bastien Tabariè S., Andrzejewski S., Pierre J.S., Jones R.G., Siegel P.M., Dong Z., Blagih J., Annis M.G., Omeroglu A., et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell Metab. 2015;22:577–589. doi: 10.1016/j.cmet.2015.08.007. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

