Histone Deacetylase Inhibitors and Phenotypical Transformation of Cancer Cells
- PMID: 30691229
- PMCID: PMC6406474
- DOI: 10.3390/cancers11020148
Histone Deacetylase Inhibitors and Phenotypical Transformation of Cancer Cells
Abstract
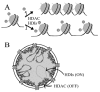
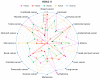
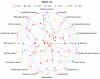
Histone deacetylase inhibitors (HDIs) are a group of potent epigenetic drugs which have been investigated for their therapeutic potential in various clinical disorders, including hematological malignancies and solid tumors. Currently, several HDIs are already in clinical use and many more are on clinical trials. HDIs have shown efficacy to inhibit initiation and progression of cancer cells. Nevertheless, both pro-invasive and anti-invasive activities of HDIs have been reported, questioning their impact in carcinogenesis. The aim of this review is to compile and discuss the most recent findings on the effect of HDIs on the epithelial-mesenchymal transition (EMT) process in human cancers. We have summarized the impact of HDIs on epithelial (E-cadherin, β-catenin) and mesenchymal (N-cadherin, vimentin) markers, EMT activators (TWIST, SNAIL, SLUG, SMAD, ZEB), as well as morphology, migration and invasion potential of cancer cells. We further discuss the use of HDIs as monotherapy or in combination with existing or novel anti-neoplastic drugs in relation to changes in EMT.
Keywords: EMT; HDAC; HDI; MET; cadherin; cancer; catenin; invasion; migration; vimentin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Grabarska A., Łuszczki J.J., Nowosadzka E., Gumbarewicz E., Jeleniewicz W., Dmoszyńska-Graniczka M., Kowalczuk K., Kupisz K., Polberg K., Stepulak A. Histone Deacetylase Inhibitor SAHA as Potential Targeted Therapy Agent for Larynx Cancer Cells. J. Cancer. 2017;8:19–28. doi: 10.7150/jca.16655. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

