Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes
- PMID: 30691231
- PMCID: PMC6406349
- DOI: 10.3390/antiox8020030
Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes
Abstract
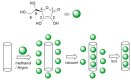
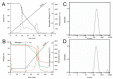
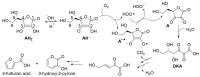
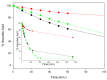
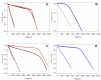
Antioxidant activity of native vitamin C (ascorbic acid, AH₂) is hampered by instability in solution. Selective loading of AH₂ into the inner lumen of natural halloysite nanotubes (HNT) yields a composite nanoantioxidant (HNT/AH₂), which was characterized and investigated for its reactivity with the persistent 1,1-diphenyl-2-picrylhydrazyl (DPPH•) radical and with transient peroxyl radicals in the inhibited autoxidation of organic substrates, both in organic solution (acetonitrile) and in buffered (pH 7.4) water in comparison with native AH₂. HNT/AH₂ showed excellent antioxidant performance being more effective than native ascorbic acid by 131% in acetonitrile and 290% (three-fold) in aqueous solution, under identical settings. Reaction with peroxyl radicals has a rate constant of 1.4 × 10⁶ M-1 s-1 and 5.1 × 10⁴ M-1 s-1, respectively, in buffered water (pH 7.4) and acetonitrile, at 30 °C. Results offer physical understanding of the factors governing HNT/AH₂ reactivity. Improved performance of HNT/AH₂ is unprecedented among forms of stabilized ascorbic acid and its relevance is discussed on kinetic grounds.
Keywords: antioxidant; ascorbic acid; biomimetic; halloysite; nanoantioxidant; nanotube; peroxyl radicals; rate constant; stability; vitamin C.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. 5th ed. Oxford University Press; Oxford, UK: 2015.
-
- Harris J.R. Ascorbic Acid: Biochemistry and Biomedical Cell Biology, in Subcellular Biochemistry. Volume 25. Springer; New York, NY, USA: 1996.
-
- Vitamin C Fact Sheet for Consumers. [(accessed on 1 July 2017)]; Available online: https://ods.od.nih.gov/factsheets/VitaminC-Consumer/
Grants and funding
LinkOut - more resources
Full Text Sources

