Metabolism and Biological Activities of 4-Methyl-Sterols
- PMID: 30691248
- PMCID: PMC6385002
- DOI: 10.3390/molecules24030451
Metabolism and Biological Activities of 4-Methyl-Sterols
Abstract
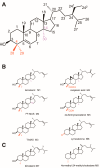
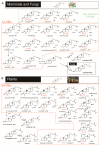
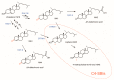
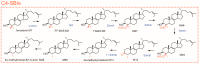
4,4-Dimethylsterols and 4-methylsterols are sterol biosynthetic intermediates (C4-SBIs) acting as precursors of cholesterol, ergosterol, and phytosterols. Their accumulation caused by genetic lesions or biochemical inhibition causes severe cellular and developmental phenotypes in all organisms. Functional evidence supports their role as meiosis activators or as signaling molecules in mammals or plants. Oxygenated C4-SBIs like 4-carboxysterols act in major biological processes like auxin signaling in plants and immune system development in mammals. It is the purpose of this article to point out important milestones and significant advances in the understanding of the biogenesis and biological activities of C4-SBIs.
Keywords: 4-methylsterol; C4-demethylation complex (C4DMC); development; genetic disease; hormone; steroid; sterol.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Phillips K.M., Ruggio D.M., Toivo J.I., Swank M.A., Simpkins A.H. Free and Esterified Sterol Composition of Edible Oils and Fats. J. Food Compost. Anal. 2002;15:123–142. doi: 10.1006/jfca.2001.1044. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

